Lab Med Online.
2015 Jul;5(3):121-126. 10.3343/lmo.2015.5.3.121.
Novel Non-contiguous Duplications in the DMD Gene in Five Patients with Duchenne Muscular Dystrophy
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. KAL1119@yuhs.ac
- 2Department of Rehabilitation, Institute of Neuromuscular Disease, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1821778
- DOI: http://doi.org/10.3343/lmo.2015.5.3.121
Abstract
- BACKGROUND
Muscular dystrophy is an X-linked recessive disorder caused by mutations in the DMD gene. Muscular dystrophy is classified into 2 types; Duchenne muscular dystrophy (DMD), which has severe clinical symptoms, and Becker muscular dystrophy (BMD), which has much milder clinical symptoms. Phenotypic progression to either DMD or BMD can be predicted by analyzing mutations in DMD by using the reading frame rule.
METHODS
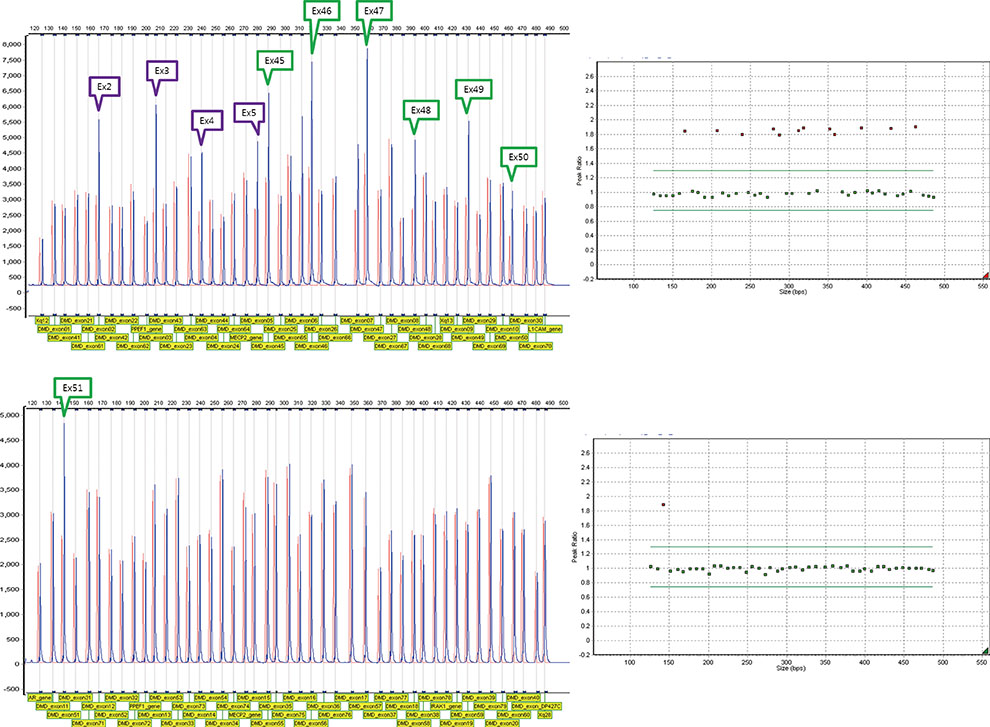
Of 88 patients with mutations in DMD, which were detected using Multiplex Ligation-dependent Probe Amplification DMD test kit (MRC-Holland, The Netherlands), medical records of 5 patients with non-contiguous duplications were reviewed. These rare non-contiguous duplications in DMD were compared with those reported previously.
RESULTS
We identified 3 novel non-contiguous duplications in DMD that included exons 2-7 and 45-51, exons 5-37 and 50-59, and exons 52-53 and 56-61. The 5 patients with these non-contiguous duplications showed the phenotypic features of DMD. Especially, duplication of exons 52-53 and 56-61 was observed in a family, i.e., 2 DMD-affected brothers and their carrier mother.
CONCLUSIONS
Prediction of phenotypes associated with complex non-contiguous duplications by using the reading frame rule is difficult because the duplications affect the expression of DMD together. Because most patients with non-contiguous duplications showed the phenotypic features of DMD, the reading frame rule should be interpreted cautiously. This study provides important insights on the non-contiguous duplications in DMD for understanding genotype-phenotype correlations and for developing dystrophin for therapeutic purposes.
Keyword
MeSH Terms
Figure
Reference
-
1. Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991; 1:19–29.
Article2. Forrest SM, Cross GS, Flint T, Speer A, Robson KJ, Davies KE. Further studies of gene deletions that cause Duchenne and Becker muscular dystrophies. Genomics. 1988; 2:109–114.
Article3. Prior TW, Bridgeman SJ. Experience and strategy for the molecular testing of Duchenne muscular dystrophy. J Mol Diagn. 2005; 7:317–326.
Article4. Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987; 50:509–517.
Article5. Hu XY, Ray PN, Murphy EG, Thompson MW, Worton RG. Duplicational mutation at the Duchenne muscular dystrophy locus: its frequency, distribution, origin, and phenotypegenotype correlation. Am J Hum Genet. 1990; 46:682–695.6. Roberts RG, Bobrow M, Bentley DR. Point mutations in the dystrophin gene. Proc Natl Acad Sci U S A. 1992; 89:2331–2335.
Article7. Lai KK, Lo IF, Tong TM, Cheng LY, Lam ST. Detecting exon deletions and duplications of the DMD gene using Multiplex Ligation-dependent Probe Amplification (MLPA). Clin Biochem. 2006; 39:367–372.
Article8. Gatta V, Scarciolla O, Gaspari AR, Palka C, De Angelis MV, Di Muzio A, et al. Identification of deletions and duplications of the DMD gene in affected males and carrier females by multiple ligation probe amplification (MLPA). Hum Genet. 2005; 117:92–98.
Article9. Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988; 2:90–95.
Article10. Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006; 34:135–144.
Article11. Tuffery-Giraud S, Béroud C, Leturcq F, Yaou RB, Hamroun D, Michel-Calemard L, et al. Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum Mutat. 2009; 30:934–945.
Article12. López-Hernández LB, Gómez-Díaz B, Bahena-Martínez E, Neri-Gómez T, Camacho-Molina A, Ruano-Calderón LA, et al. A novel noncontiguous duplication in the DMD gene escapes the 'reading-frame rule'. J Genet. 2014; 93:225–229.
Article13. Janssen B, Hartmann C, Scholz V, Jauch A, Zschocke J. MLPA analysis for the detection of deletions, duplications and complex rearrangements in the dystrophin gene: potential and pitfalls. Neurogenetics. 2005; 6:29–35.
Article14. Imoto N, Arinami T, Hamano K, Matsumura K, Yamada H, Hamaguchi H, et al. Topographic pattern of the rearrangement of the dystrophin gene in Japanese Duchenne muscular dystrophy. Hum Genet. 1993; 92:533–536.
Article15. White SJ, Aartsma-Rus A, Flanigan KM, Weiss RB, Kneppers AL, Lalic T, et al. Duplications in the DMD gene. Hum Mutat. 2006; 27:938–945.16. Zhang Z, Takeshima Y, Awano H, Nishiyama A, Okizuka Y, Yagi M, et al. Tandem duplications of two separate fragments of the dystrophin gene in a patient with Duchenne muscular dystrophy. J Hum Genet. 2008; 53:215–219.
Article17. Fenollar-Cortés M, Gallego-Merlo J, Trujillo-Tiebas MJ, Lorda-Sánchez I, Ayuso C. Two non-contiguous duplications in the DMD gene in a Spanish family. J Neurogenet. 2008; 22:93–101.18. Hu XY, Ray PN, Worton RG. Mechanisms of tandem duplication in the Duchenne muscular dystrophy gene include both homologous and nonhomologous intrachromosomal recombination. EMBO J. 1991; 10:2471–2477.
Article19. Lopes J, Tardieu S, Silander K, Blair I, Vandenberghe A, Palau F, et al. Homologous DNA exchanges in humans can be explained by the yeast double-strand break repair model: a study of 17p11.2 rearrangements associated with CMT1A and HNPP. Hum Mol Genet. 1999; 8:2285–2292.
Article20. Helderman-van den Enden AT, de Jong R, den Dunnen JT, Houwing-Duistermaat JJ, Kneppers AL, Ginjaar HB, et al. Recurrence risk due to germ line mosaicism: Duchenne and Becker muscular dystrophy. Clin Genet. 2009; 75:465–472.
Article21. Sommer SS. Recent human germ-line mutation: inferences from patients with hemophilia B. Trends Genet. 1995; 11:141–147.
Article22. Helmrich A, Ballarino M, Tora L. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol Cell. 2011; 44:966–977.
Article23. Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987; 51:919–928.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Female Carriers of Duchenne Muscular Dystrophy
- Acute Cerebral Infarction and Epilepsy in Duchenne Muscular Dystrophy
- Whole Exome Sequencing of a Patient with Duchenne Muscular Dystrophy
- A clinical study on Duchenne muscular dystrophy
- Sympathetic Skin Response in Patients with Duchenne Muscular Dystrophy