J Korean Surg Soc.
2011 Apr;80(4):251-259. 10.4174/jkss.2011.80.4.251.
Migrating motor complex changes after side-to-side ileal bypass in mouse ileum ex-vivo: mechanism underlying the blind loop syndrome?
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. kjparkmd@plaza.snu.ac.kr
- 2Department of Physiology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1820015
- DOI: http://doi.org/10.4174/jkss.2011.80.4.251
Abstract
- PURPOSE
This study was intended to investigate the migrating motor complex (MMC) changes after ileal bypass in ex-vivo mouse models.
METHODS
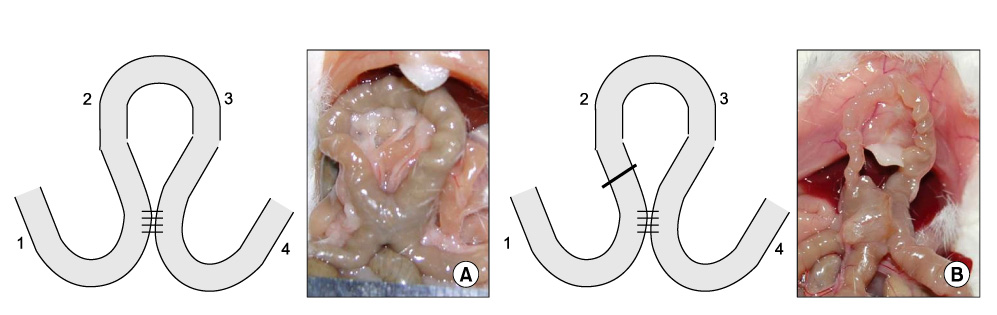
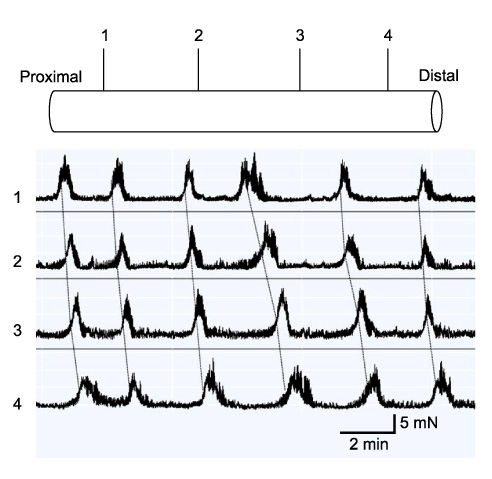
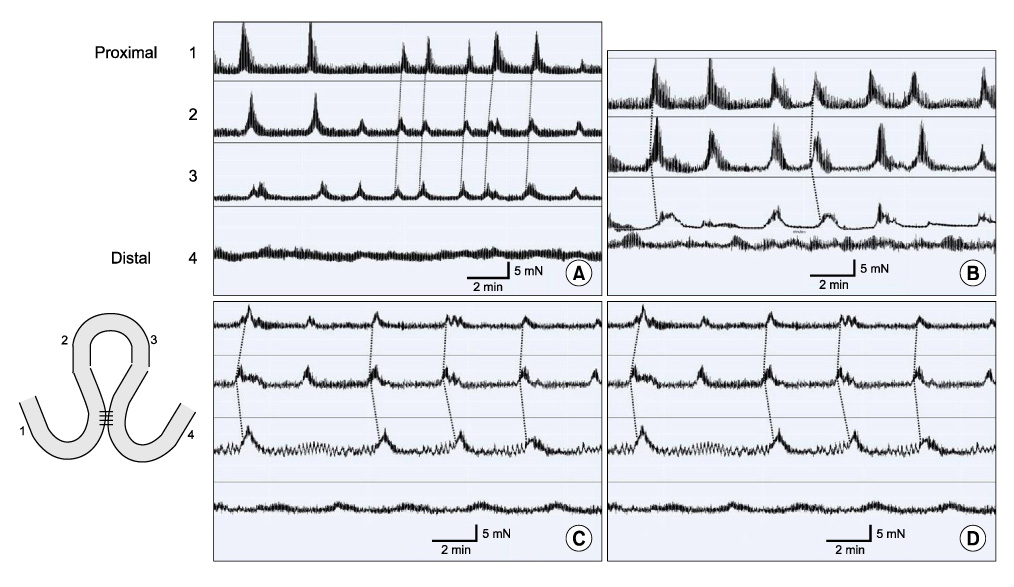
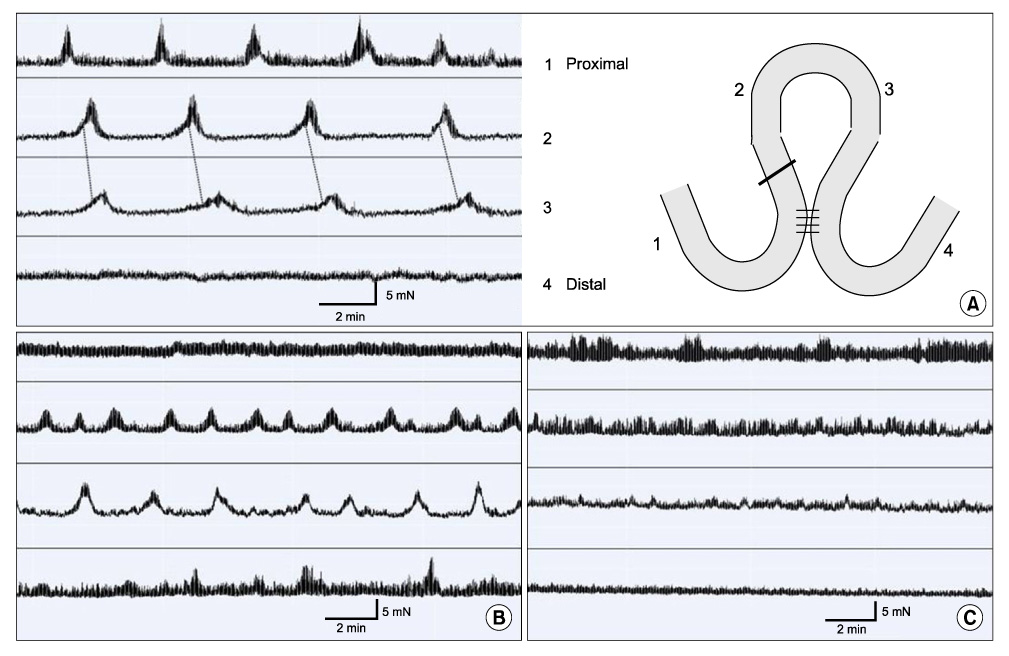
Partial (side-to-side) and total bypass (occlusion of proximal part of bypassed loop) were performed on ileums of female Institute of Cancer Research mice. After 2 and 4 weeks, the bypassed segments were harvested and MMCs were recorded at 4 different sites ex-vivo. Amplitude, duration, interval, direction of propagation, and the area under the curve (AUC) of MMCs were measured and compared to those of the controls.
RESULTS
In control mice (n = 7), most MMCs propagated aborally (91.1%). After 2 weeks of partial bypass (n = 4), there was a significant decrease in both amplitude and AUC, and orally-propagating MMCs increased significantly (45%, P = 0.002). Bidirectional MMCs (originating in the bypassed loop and propagating in both directions) were also observed (10%). The amplitude of the MMCs remained decreased at 4 weeks after partial bypass (n = 4), and neither the AUC nor the direction of propagation showed significant changes compared to 2 weeks. Similarly, in the total bypass model, both the amplitude and AUC of the MMCs decreased significantly compared to controls. In contrast to partial bypass, 95% of the MMCs within the bypassed loop propagated aborally after 2 weeks (n = 6), which was similar to the control state. After 4 weeks (n = 5), however, MMCs either lost their temporal relationship or completely disappeared.
CONCLUSION
The changes in propagation direction of the MMCs in the partially bypassed loop may contribute to stagnation of bowel contents and the development of blind loop syndrome.
Keyword
MeSH Terms
Figure
Reference
-
1. Botsford TW, Gazzaniga AB. Blind pouch syndrome. A complication of side to side intestinal anastomosis. Am J Surg. 1967. 113:486–490.2. Adachi Y, Matsushima T, Mori M, Sugimachi K, Oiwa T. Blind loop syndrome: multiple ileal ulcers following side-to-side anastomosis. Pathology. 1993. 25:402–404.3. Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992. 216:269–278.4. Wingate DL. Backwards and forwards with the migrating complex. Dig Dis Sci. 1981. 26:641–666.5. Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985. 89:894–913.6. Wood JD. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am J Dig Dis. 1973. 18:477–488.7. Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000. 84:162–168.8. Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997. 9:99–107.9. Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001. 536(Pt 2):555–568.10. Yanagida H, Yanase H, Sanders KM, Ward SM. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004. 127:1748–1759.11. Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977. 59:1158–1166.12. Moon SH, Oh HK, Ryoo S, Choe EK, Moon JS, Park KJ. Changes in migrating motor complex after bowel obstruction in the murine ileum. J Korean Soc Coloproctol. 2010. 26:171–178.13. Galligan JJ, Furness JB, Costa M. Migration of the myoelectric complex after interruption of the myenteric plexus: intestinal transection and regeneration of enteric nerves in the guinea pig. Gastroenterology. 1989. 97:1135–1146.14. Powell AK, O'brien SD, Fida R, Bywater RA. Neural integrity is essential for the propagation of colonic migrating motor complexes in the mouse. Neurogastroenterol Motil. 2002. 14:495–504.15. Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol. 2001. 1:604–610.16. Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, et al. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006. 290:C1411–C1427.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alcoholic Type Cirrhosis Following Side to Side Ileo-Transverse Colon Anastomosis
- Changes in Migrating Motor Complex after Bowel Obstruction in the Murine Ileum
- A Case of Ileal Duplication with Intestinal Hemorrhage
- Ileal Loop Cutaneous Urinary Diversion: A Clinical Review on 8 Cases
- Electrophysiological and Mechanical Characteristics in Human Ileal Motility: Recordings of Slow Waves Conductions and Contractions, In vitro