J Clin Neurol.
2005 Oct;1(2):142-147. 10.3988/jcn.2005.1.2.142.
Plasma Fibrinolysis Inhibitor Levels in Acute Stroke Patients with Thrombolysis Failure
- Affiliations
-
- 1Department of Neurology, National Core Research Center for Nanomedical Technology, Brain Research Institute, Yonsei University College of Medicine, Korea. jhheo@yumc.yonsei.ac.kr
- 2Department of Diagnostic Radiology, National Core Research Center for Nanomedical Technology, Brain Research Institute, Yonsei University College of Medicine, Korea.
- 3Department of Neurology, Yonsei University Wonju College of Medicine, Korea.
- 4Department of Neurology, Sanggye Paik Hospital, Korea.
- 5Department of Neurology, Yonsei University College of Medicine, Yongdong Severance Hospital, Seoul, Korea.
- KMID: 1808484
- DOI: http://doi.org/10.3988/jcn.2005.1.2.142
Abstract
- BACKGROUND AND PURPOSE
Thrombolytics-induced recanalization fails in a significant portion of patients with ischemic stroke, which is partly due to the resistance of clots to lysis by thrombolytic agents. The pretreatment level of endogenous fibrinolysis inhibitors may affect such thrombolysis failure.
METHODS
We studied 43 stroke patients whose arterial recanalization had been evaluated by angiography, and whose blood had been obtained prior to the administration of thrombolytic agents. Plasma samples from 34 healthy volunteers were used as normal controls. Plasminogen activator inhibitor type 1 (PAI-1) and thrombin-activatable fibrinolysis inhibitor (TAFI) levels were quantified using an enzyme-linked immunosorbent assay.
RESULTS
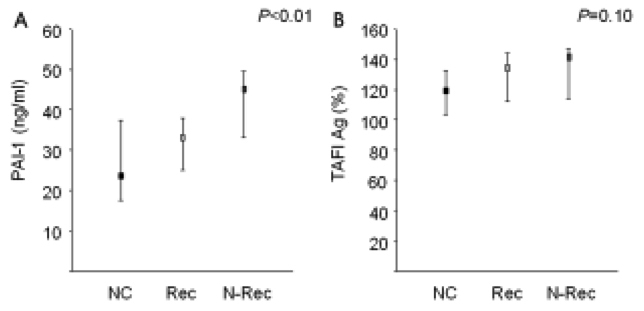
Arteries were recanalized [Thrombolysis in Myocardial Infarction (TIMI) grade 2 or 3] in 30 patients, but not (TIMI grade 0 or 1) in the other 13. The plasma PAI-1 level was significantly higher in patients without recanalization (nonrecanalization) than in those with recanalization and in normal controls. The TAFI levels did not differ among the groups.
CONCLUSIONS
The pretreatment PAI-1 levels are increased in acute stroke patients with thrombolysis failure.
Keyword
MeSH Terms
-
Angiography
Arteries
Carboxypeptidase U
Enzyme-Linked Immunosorbent Assay
Fibrinolysis*
Fibrinolytic Agents
Healthy Volunteers
Humans
Myocardial Infarction
Plasma*
Plasminogen Activator Inhibitor 1
Plasminogen Activators
Stroke*
Carboxypeptidase U
Fibrinolytic Agents
Plasminogen Activator Inhibitor 1
Plasminogen Activators
Figure
Reference
-
1. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.2. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999. 282:2003–2011.
Article3. Mohr J, Choi D, Grotta J, Weir B, Wolf PA. Stroke: Pathophysiology, diagnosis, and management. 2004. Philadelphia: Churchill Livingstone.4. Heo JH, Kim SH, Lee KY, Kim EH, Chu CK, Nam JM. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke. 2003. 34:e48–e50.
Article5. Ribo M, Montaner J, Molina CA, Arenillas JF, Santamarina E, Alvarez-Sabin J. Admission fibrinolytic profile predicts clot lysis resistance in stroke patients treated with tissue plasminogen activator. Thromb Haemost. 2004. 91:1146–1151.
Article6. Abciximab Emergent Stroke Treatment Trial (AbESTT) Investigators. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke. 2005. 36:880–890.7. Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005. 36:1432–1438.
Article8. Sinkovic A. Pretreatment plasminogen activator inhibitor-1 (PAI-1) levels and the outcome of thrombolysis with streptokinase in patients with acute myocardial infarction. Am Heart J. 1998. 136:406–411.
Article9. Heo JH, Lee KY, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology. 2003. 60:1684–1687.
Article10. Lee KY, Kim DI, Kim SH, Lee SI, Chung HW, Shim YW, et al. Sequential combination of intravenous recombinant tissue plasminogen activator and intra-arterial urokinase in acute ischemic stroke. Am J Neuroradiol. 2004. 25:1470–1475.11. Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, et al. Thrombolysis in Myocardial Infarction (TIMI) trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987. 76:142–154.
Article12. Rupin A, Martin F, Vallez MO, Bonhomme E, Verbeuren TJ. Inactivation of plasminogen activator inhibitor-1 accelerates thrombolysis of a platelet-rich thrombus in rat mesenteric arterioles. Thromb Haemost. 2001. 86:1528–1531.
Article13. Lindgren A, Lindoff C, Norrving B, Astedt B, Johansson BB. Tissue plasminogen activator and plasminogen activator inhibitor-1 in stroke patients. Stroke. 1996. 27:1066–1071.
Article14. Fisher M, Francis R. Altered coagulation in cerebral ischemia. Platelet, thrombin, and plasmin activity. Arch Neurol. 1990. 47:1075–1079.
Article15. Wang J, Li J, Liu Q. Association between platelet activation and fibrinolysis in acute stroke patients. Neurosci Lett. 2005. 384:305–309.
Article16. Dobrovolsky AB, Titaeva EV. The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochemistry (Mosc). 2002. 67:99–108.17. Huber K. Plasminogen activator inhibitor type-1 (part one): basic mechanisms, regulation, and role for thromboembolic disease. J Thromb Thrombolysis. 2001. 11:183–193.18. Jang IK, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, et al. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989. 79:920–928.
Article19. Cannon CP. Overcoming thrombolytic resistance: rationale and initial clinical experience combining thrombolytic therapy and glycoprotein IIb/IIIa receptor inhibition for acute myocardial infarction. J Am Coll Cardiol. 1999. 34:1395–1402.20. Levi M, Biemond BJ, van Zonneveld AJ, ten Cate JW, Pannekoek H. Inhibition of plasminogen activator inhibitor-1 activity results in promotion of endogenous thrombolysis and inhibition of thrombus extension in models of experimental thrombosis. Circulation. 1992. 85:305–312.
Article21. Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999. 99:3050–3055.
Article22. Juhan-Vague I, Morange PE, Aubert H, Henry M, Aillaud MF, Alessi MC, et al. Plasma thrombin-activatable fibrinolysis inhibitor antigen concentration and genotype in relation to myocardial infarction in the north and south of Europe. Arterioscler Thromb Vasc Biol. 2002. 22:867–873.
Article23. Morange PE, Juhan-Vague I, Scarabin PY, Alessi MC, Luc G, Arveiler D, et al. Association between TAFI antigen and Ala147Thr polymorphism of the TAFI gene and the angina pectoris incidence. The PRIME study (Prospective Epidemiological Study of MI). Thromb Haemost. 2003. 89:554–560.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coagulation-Fibrinolysis system in acute progressive and non-progressive stroke: Preliminary study
- Interventional Recanalization Treatment of Acute Ischemic Stroke
- Plasma Level of IL-6 and Its Relationship to Procoagulant and Fibrinolytic Markers in Acute Ischemic Stroke
- The Role of the Coagulation and Fibrinolytic Pathway in Acute Lung Injury
- The Significance of Thrombin Activable Fibrinolysis Inhibitor in Ischemic Stroke