Allergy Asthma Immunol Res.
2011 Jul;3(3):199-204. 10.4168/aair.2011.3.3.199.
The Role of Plasmacytoid and Myeloid Dendritic Cells in Induction of Asthma in a Mouse Model and the Effect of a TLR9 Agonist on Dendritic Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Dankook University College of Medicine, Cheonan, Korea. jihunmo@gmail.com
- 2Department of Medicine, University of California, San Diego, La Jolla, CA, USA.
- KMID: 1803326
- DOI: http://doi.org/10.4168/aair.2011.3.3.199
Abstract
- PURPOSE
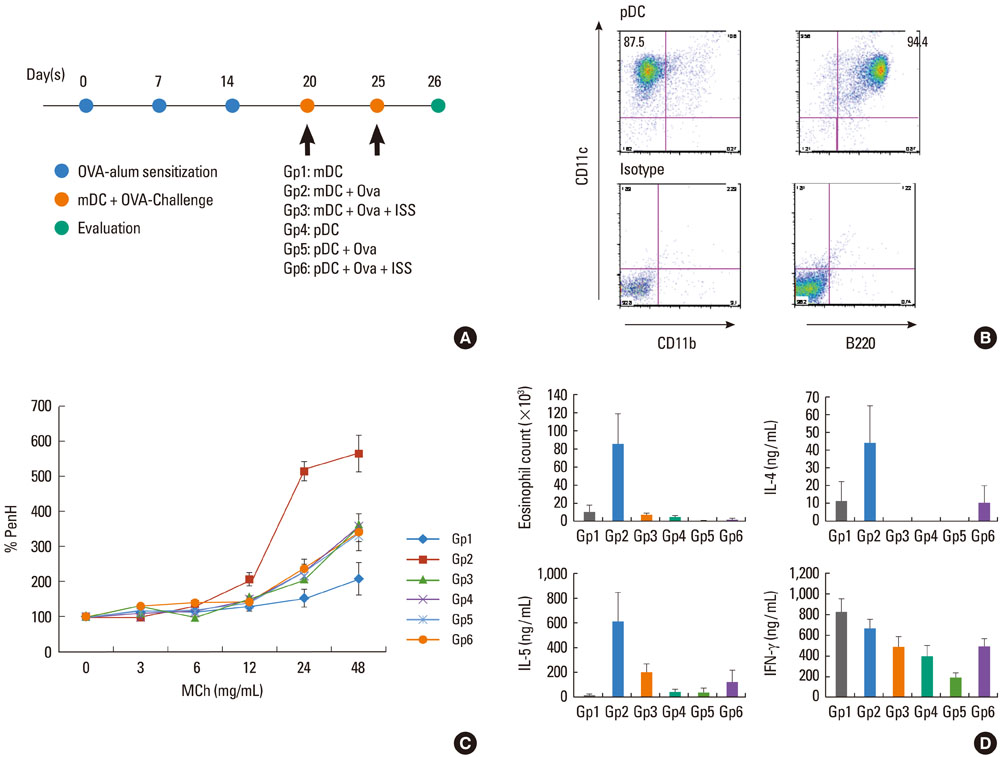
To determine the role of plasmacytoid dendritic cells (pDC) and myeloid dendritic cells (mDC) in priming effector T cells to induce allergy, and to evaluate the effect of immunostimulatory sequences (ISS, TLR9 agonist) on dendritic cells.
METHODS
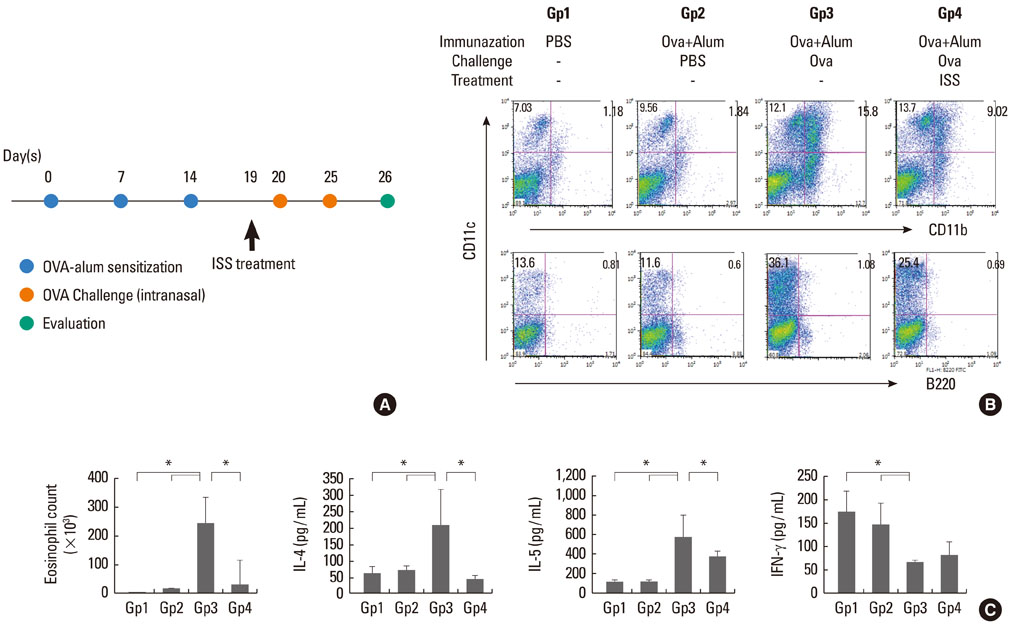
Cultured mDC and pDC with/without ISS were injected intratracheally into sensitized Balb/C mice. Mice were sacrificed, and then pulmonary function tests, bronchoalveolar lavage (BAL), cell counts, and cytokine levels were evaluated. Migration of dendritic cells was also evaluated after ISS administration.
RESULTS
In mice injected with mDC, airway hyperresponsiveness, eosinophil counts, and Th2 cytokine levels in BAL increased with increasing numbers of mDC injected. However, in mice injected with pDC, none of these changed, suggesting poor priming of T cells by pDC. In addition, mDC pulsed with ISS inhibited asthmatic reactions, and ISS administration inhibited migration of DC to the lung.
CONCLUSIONS
We suggest that pDC played a limited role in priming T cells in this asthma model and that mDC played a major role in inducing asthma. In addition, ISS inhibited migration of DC to the lung.
Keyword
MeSH Terms
Figure
Reference
-
1. Drazen JM, Arm JP, Austen KF. Sorting out the cytokines of asthma. J Exp Med. 1996. 183:1–5.2. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992. 326:298–304.3. Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996. 183:109–117.4. Garlisi CG, Falcone A, Billah MM, Egan RW, Umland SP. T cells are the predominant source of interleukin-5 but not interleukin-4 mRNA expression in the lungs of antigen-challenged allergic mice. Am J Respir Cell Mol Biol. 1996. 15:420–428.5. Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000. 106:551–559.6. Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007. 13:552–559.7. Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999. 223:77–92.8. Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001. 193:51–60.9. Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998. 161:7054–7062.10. Broide DH, Stachnick G, Castaneda D, Nayar J, Miller M, Cho JY, Roman M, Zubeldia J, Hayashi T, Raz E. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of Th2 responses in a mouse model of asthma. J Clin Immunol. 2001. 21:175–182.11. Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Brière F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001. 2:1144–1150.12. Nakano H, Yanagita M, Gunn MD. CD11c(+) B220(+) Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001. 194:1171–1178.13. Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997. 185:1101–1111.14. Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999. 283:1183–1186.15. de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004. 200:89–98.16. Abe K, Nguyen KP, Fine SD, Mo JH, Shen C, Shenouda S, Corr M, Jung S, Lee J, Eckmann L, Raz E. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci U S A. 2007. 104:17022–17027.