Yonsei Med J.
2013 Nov;54(6):1336-1341. 10.3349/ymj.2013.54.6.1336.
Inflammatory Responses in the Muscle Coat of Stomach and Small Bowel in the Postoperative Ileus Model of Guinea Pig
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. HJPARK21@yuhs.ac
- 2Department of Physiology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Medical Science, Medical School, McMaster University, Ontario, Canada.
- KMID: 1798128
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1336
Abstract
- PURPOSE
Small intestinal function returns first after surgery, and then the function of the stomach returns to normal after postoperative ileus (POI). The aim of this study was to investigate inflammatory responses in the muscle coat of stomach and small intestine in guinea pig POI model.
MATERIALS AND METHODS
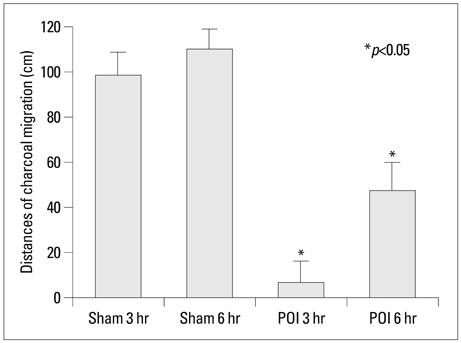
The distance of charcoal migration from pylorus to the distal intestine was measured. Hematoxylin and eosin (H&E) and immunohistochemical stain for calprotectin were done from the histologic sections of stomach, jejunum and ileum obtained at 3 and 6 hour after operation. Data were compared between sham operation and POI groups.
RESULTS
The distance of charcoal migration was significantly reduced in the 3 and 6 hour POI groups compared with sham operated groups (p<0.05). On H&E staining, the degree of inflammation was significantly higher in the stomach of 3 hour POI groups compared with jejunum and ileum of POI groups or sham operated groups (p<0.05). Calprotectin positive cells were significantly increased in the muscle coat of stomach of 3 hour POI groups compared with jejunum and ileum of POI groups or sham operated groups (p<0.05). There was strong association between the degree of inflammation and calprotectin positive cells in stomach.
CONCLUSION
Postoperative ileus induced by cecal manipulation significantly increased the degree of inflammation and calprotectin positive cells in the muscle coat of stomach as a remote organ. The relevance of degree of inflammation and the recovery time of ileus should be pursued in the future research.
Keyword
MeSH Terms
Figure
Reference
-
1. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000; 87:1480–1493.
Article2. Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. 2001; 182:5A Suppl. 3S–10S.
Article3. Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg. 1999; 230:251–259.
Article4. Longo WE, Virgo KS, Johnson FE, Oprian CA, Vernava AM, Wade TP, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000; 43:83–91.
Article5. Lim HC, Chon NR, Choi EJ, Lee YH, Lee MG, Park H. The effect of itopride hydrochloride on the gastrointestinal motility in postoperative ileus of guinea pigs. Korean J Neurogastroenterol Motil. 2008; 14:18–23.6. Kalff JC, Türler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, et al. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003; 237:301–315.
Article7. Kehlet H. Postoperative ileus. Gut. 2000; 47:Suppl 4. iv85–iv86.
Article8. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998; 228:652–663.
Article9. Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999; 117:378–387.
Article10. Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000; 118:316–327.
Article11. Zwadlo G, Brüggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988; 72:510–515.12. Bhardwaj RS, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, et al. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol. 1992; 22:1891–1897.
Article13. Lagasse E, Weissman IL. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992; 79:1907–1915.
Article14. The FO, Bennink RJ, Ankum WM, Buist MR, Busch OR, Gouma DJ, et al. Intestinal handling-induced mast cell activation and inflammation in human postoperative ileus. Gut. 2008; 57:33–40.
Article15. Kim HS, Choi EJ, Park H. The effect of mosapride citrate on proximal and distal colonic motor function in the guinea-pig in vitro. Neurogastroenterol Motil. 2008; 20:169–176.
Article16. de Jonge WJ, van den Wijngaard RM, The FO, ter Beek ML, Bennink RJ, Tytgat GN, et al. Postoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in mice. Gastroenterology. 2003; 125:1137–1147.
Article17. Schwarz NT, Kalff JC, Türler A, Speidel N, Grandis JR, Billiar TR, et al. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology. 2004; 126:159–169.
Article18. McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008; 60:63–70.
Article19. Kendall RA, Alhnan MA, Nilkumhang S, Murdan S, Basit AW. Fabrication and in vivo evaluation of highly pH-responsive acrylic microparticles for targeted gastrointestinal delivery. Eur J Pharm Sci. 2009; 37:284–290.
Article20. Merchant HA, McConnell EL, Liu F, Ramaswamy C, Kulkarni RP, Basit AW, et al. Assessment of gastrointestinal pH, fluid and lymphoid tissue in the guinea pig, rabbit and pig, and implications for their use in drug development. Eur J Pharm Sci. 2011; 42:3–10.
Article21. Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000; 165:5069–5076.
Article22. Mizuno S, Kanai T, Mikami Y, Sujino T, Ono Y, Hayashi A, et al. CCR9+ plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol Lett. 2012; 146:64–69.
Article23. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005; 6:844–851.
Article24. Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008; 20:Suppl 1. 81–90.
Article25. Schwarz NT, Beer-Stolz D, Simmons RL, Bauer AJ. Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis. Ann Surg. 2002; 235:31–40.26. Türler A, Schnurr C, Nakao A, Tögel S, Moore BA, Murase N, et al. Endogenous endotoxin participates in causing a panenteric inflammatory ileus after colonic surgery. Ann Surg. 2007; 245:734–744.
Article27. Limburg PJ, Ahlquist DA, Sandborn WJ, Mahoney DW, Devens ME, Harrington JJ, et al. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000; 95:2831–2837.
Article28. Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003; 35:642–647.
Article29. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006; 12:524–534.
Article30. Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991; 444:743–761.
Article31. van der Vliet A, Tuinstra TJ, Bast A. Modulation of oxidative stress in the gastrointestinal tract and effect on rat intestinal motility. Biochem Pharmacol. 1989; 38:2807–2818.
Article32. Petitclerc E, Levesque L, Grose JH, Poubelle PE, Marceau F. Pathologic leukocyte infiltration of the rabbit aorta confers a vasomotor effect to chemotactic peptides through cyclooxygenase-derived metabolites. J Immunol. 1996; 156:3426–3434.33. Prasad M, Matthews JB. Deflating postoperative ileus. Gastroenterology. 1999; 117:489–492.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "Inflammatory Responses in the Muscle Coat of Stomach and Small Bowel in the Postoperative Ileus Model of Guinea Pig" by Choi HK, et al. (Yonsei Med J 2013;54:1336-41.)
- Inflammation, Impaired Motility, and Permeability in a Guinea Pig Model of Postoperative Ileus
- The Effect of DA-9701 in Opioid-induced Bowel Dysfunction of Guinea Pig
- The Effects of Heparin and Protamine on Contraction of Tracheal Smooth Muscle Induced by Carbachol in the Guinea Pig
- Familial Trichophyton mentagrophytes Infection Transmitted from Guinea Pig