J Korean Med Sci.
2014 Jun;29(6):846-851. 10.3346/jkms.2014.29.6.846.
Evaluation of Stem Cell Components in Retrocorneal Membranes
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University Hospital, College of Medicine, Seoul, Korea. jck50ey@kornet.net
- 2Department of Pathology, Chung-Ang University Hospital, College of Medicine, Seoul, Korea.
- KMID: 1796949
- DOI: http://doi.org/10.3346/jkms.2014.29.6.846
Abstract
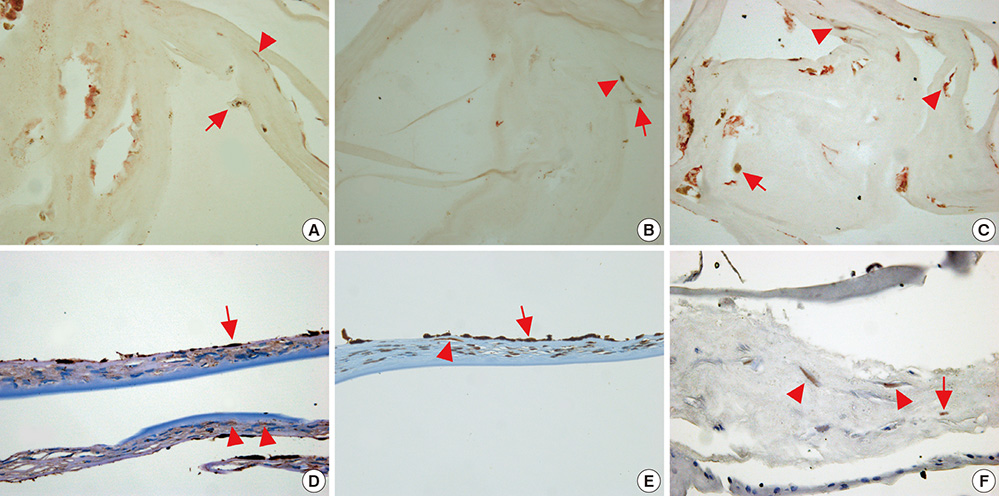
- The purpose of this study was to elucidate the origin and cellular composition of retrocorneal membranes (RCMs) associated with chemical burns using immunohistochemical staining for primitive cell markers. Six cases of RCMs were collected during penetrating keratoplasty. We examined RCMs with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) staining and immunohistochemical analysis using monoclonal antibodies against hematopoietic stem cells (CD34, CD133, c-kit), mesenchymal stem cells (beta-1-integrin, TGF-beta, vimentin, hSTRO-1), fibroblasts (FGF-beta, alpha-smooth muscle actin), and corneal endothelial cells (type IV collagen, CD133, VEGF, VEGFR1). Histologic analysis of RCMs revealed an organized assembly of spindle-shaped cells, pigment-laden cells, and thin collagenous matrix structures. RCMs were positive for markers of mesenchymal stem cells including beta-1-integrin, TGF-beta, vimentin, and hSTRO-1. Fibroblast markers were also positive, including FGF-beta and alpha-smooth muscle actin (SMA). In contrast, immunohistochemical staining was negative for hematopoietic stem cell markers including CD34, CD133 and c-kit as well as corneal endothelial cell markers such as type IV collagen, CD133 except VEGF and VEGFR1. Pigment-laden cells did not stain with any antibodies. The results of this study suggest that RCMs consist of a thin collagen matrix and fibroblast-like cells and may be a possible neogenetic structure produced from a lineage of bone marrow-derived mesenchymal stem cells.
MeSH Terms
-
Adult
Aged
Antigens, CD/metabolism
Cornea/*cytology/pathology
Cytokines/metabolism
Endothelial Cells/cytology/metabolism
Female
Fibroblasts/cytology/metabolism
Hematopoietic Stem Cells/cytology/metabolism
Humans
Immunohistochemistry
Intercellular Signaling Peptides and Proteins/metabolism
Male
Mesenchymal Stromal Cells/cytology/metabolism
Middle Aged
Stem Cells/cytology/*metabolism
Antigens, CD
Cytokines
Intercellular Signaling Peptides and Proteins
Figure
Reference
-
1. Kawaguchi R, Saika S, Wakayama M, Ooshima A, Ohnishi Y, Yabe H. Extracellular matrix components in a case of retrocorneal membrane associated with syphilitic interstitial keratitis. Cornea. 2001; 20:100–103.2. Rodrigues MM, Waring GO, Laibson PR, Weinreb S. Endothelial alterations in congenital corneal dystrophies. Am J Ophthalmol. 1975; 80:678–689.3. Brown SI, Kitano S. Pathogenesis of the retrocorneal membrane. Arch Ophthalmol. 1966; 75:518–525.4. Leung EW, Rife L, Smith RE, Kay EP. Extracellular matrix components in retrocorneal fibrous membrane in comparison to corneal endothelium and Descemet's membrane. Mol Vis. 2000; 6:15–23.5. Cockerham GC, Hidayat AA. Retrocorneal membrane with myofibroblasts after perforating injury: an immunohistochemical and ultrastructural study of 11 cases. Cornea. 1999; 18:700–706.6. Kay EP, Gu X, Smith RE. Corneal endothelial modulation: bFGF as direct mediator and corneal endothelium modulation factor as inducer. Invest Ophthalmol Vis Sci. 1994; 35:2427–2435.7. López-García JS, Rivas Jara L, García-Lozano I, Murube J. Histopathologic limbus evolution after alkaline burns. Cornea. 2007; 26:1043–1048.8. Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W, Jiang MH, Kim JC, Son Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009; 15:425–435.9. Lee JK, Song YS, Ha HS, Park JH, Kim MK, Park AJ, Kim JC. Endothelial progenitor cells in pterygium pathogenesis. Eye (Lond). 2007; 21:1186–1193.10. Polisetty N, Fatima A, Madhira SL, Sangwan VS, Vemuganti GK. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008; 14:431–442.11. Kremer I, Rapuano CJ, Cohen EJ, Laibson PR, Eagle RC Jr. Retrocorneal fibrous membranes in failed corneal grafts. Am J Ophthalmol. 1993; 115:478–483.12. Sanchez J, Polack FM. Autoradiographic study of retrocorneal membranes. Ann Ophthalmol. 1978; 10:1547–1552.13. Kay ED, Cheung CC, Jester JV, Nimni ME, Smith RE. Type I collagen and fibronectin synthesis by retrocorneal fibrous membrane. Invest Ophthalmol Vis Sci. 1982; 22:200–212.14. Dogru M, Kato N, Matsumoto Y, Tanaka Y, Akabane N, Shimmura S, Tsubota K, Shimazaki J. Immunohistochemistry and electron microscopy of retrocorneal scrolls in syphilitic interstitial keratitis. Curr Eye Res. 2007; 32:863–870.15. Chiou AG, Chang C, Kaufman SC, Ohta T, Maitchouk D, Beuerman RW, Kaufman HE. Characterization of fibrous retrocorneal membrane by confocal microscopy. Cornea. 1998; 17:669–671.16. Jakobiec FA, Bhat P. Retrocorneal membranes: a comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol. 2010; 150:230–242.e2.17. Ide T, Nishida K, Yamato M, Sumide T, Utsumi M, Nozaki T, Kikuchi A, Okano T, Tano Y. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials. 2006; 27:607–614.18. Toti P, Tosi GM, Traversi C, Schürfeld K, Cardone C, Caporossi A. CD-34 stromal expression pattern in normal and altered human corneas. Ophthalmology. 2002; 109:1167–1171.19. Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006; 83:3–15.20. Lee IG, Ye J, Kim JC. The involvement of multipotential progenitor cells in Mooren's ulcer. Yonsei Med J. 2005; 46:353–358.21. Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells: biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007; 9:204.22. Aoki H, Hara A, Motohashi T, Chem H, Kunisada T. Iris as a recipient tissue for pigment cells: organized in vivo differentiation of melanocytes and pigmented epithelium derived from embryonic stem cells in vitro. Dev Dyn. 2008; 237:2394–2404.23. Asami M, Sun G, Yamaguchi M, Kosaka M. Multipotent cells from mammalian iris pigment epithelium. Dev Biol. 2007; 304:433–446.24. Sun G, Asami M, Ohta H, Kosaka J, Kosaka M. Retinal stem/progenitor properties of iris pigment epithelial cells. Dev Biol. 2006; 289:243–252.25. Wunderlich K, Senn BC, Reiser P, Pech M, Flammer J, Meyer P. Connective tissue growth factor in retrocorneal membranes and corneal scars. Ophthalmologica. 2000; 214:341–346.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Argon Laser Photocoagulation Combined With Subconjunctival and Intravitreal Bevacizumab Injections for a Retrocorneal Neovascular Membrane
- Immunohistochemical Study of Proliferative Vitreoretinal Membranes
- Cellular components of proliferative vitreoretinal membranes
- Observations on iris melanocytes implanted in the cornea
- Regulatory issues in stem cell therapeutics in Korea: efficacy or efficiency?