J Korean Med Sci.
2014 Jan;29(1):76-83. 10.3346/jkms.2014.29.1.76.
Correction of Anemia with Continuous Erythropoietin Receptor Activator in Korean Patients on Long-Term Hemodialysis
- Affiliations
-
- 1Department of Internal Medicine, Hallym University College of Medicine, Hallym Kidney Research Institute, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. skimim@snu.ac.kr
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Internal Medicine, Gachon University College of Medicine, Incheon, Korea.
- 5Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, The Catholic University of Korea School of Medicine, Seoul, Korea.
- KMID: 1796918
- DOI: http://doi.org/10.3346/jkms.2014.29.1.76
Abstract
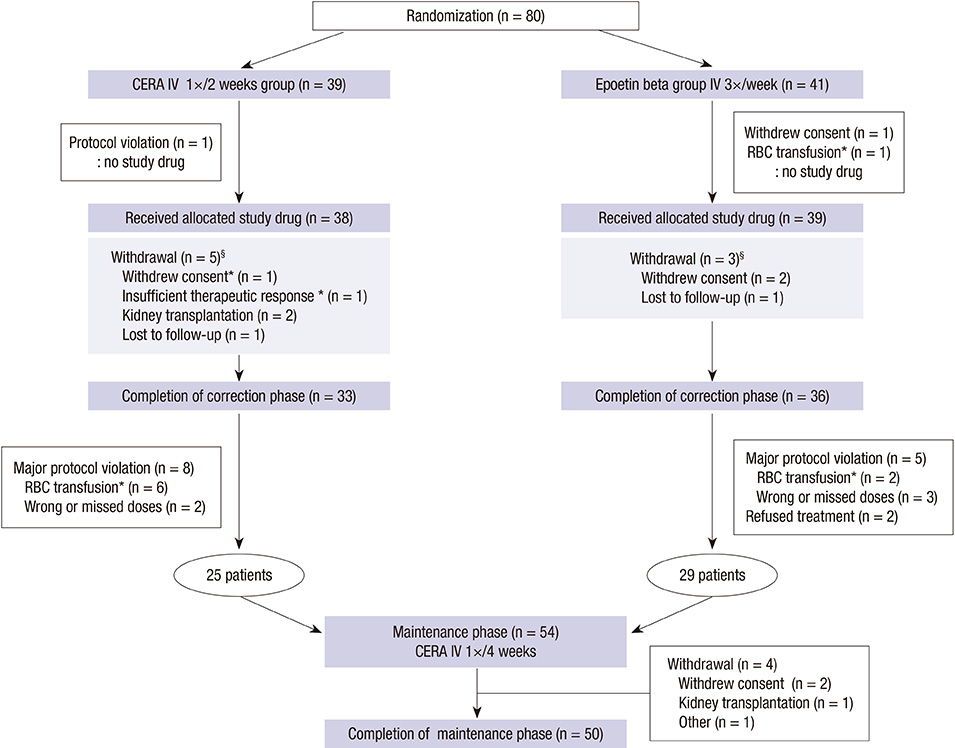
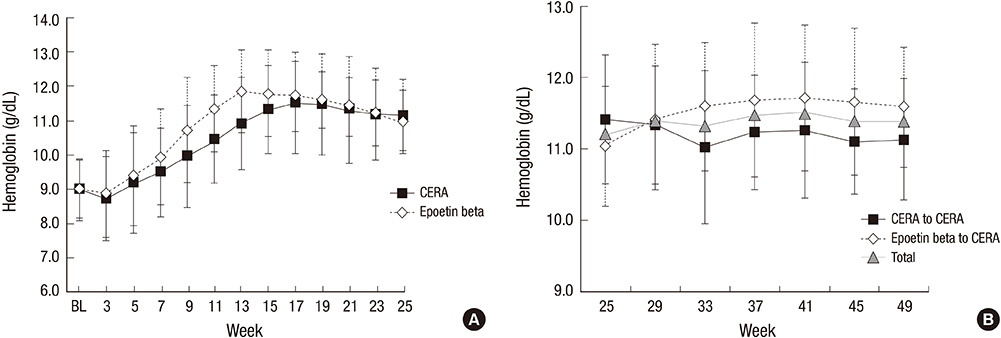
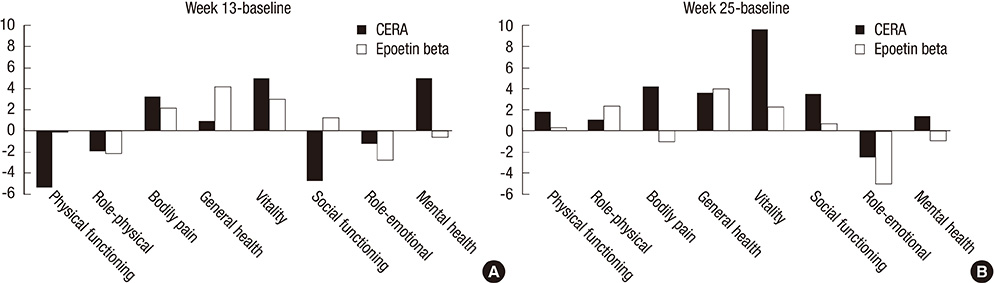
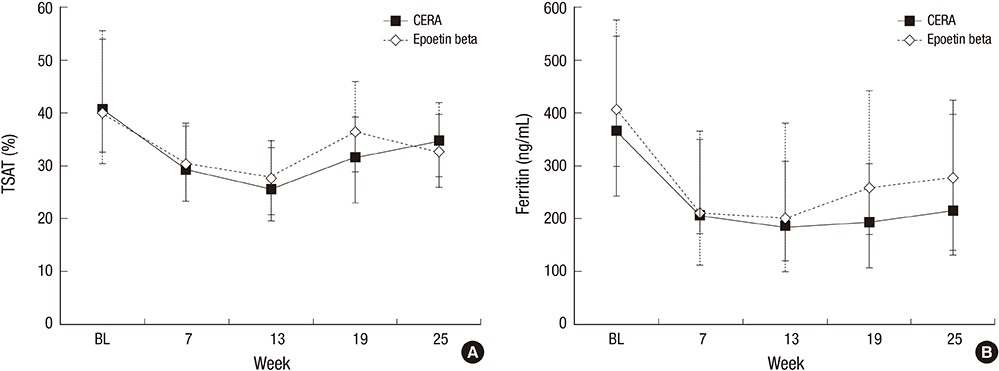
- Continuous erythropoietin receptor activator (CERA) is an erythropoietin with a long-half life. This study investigated the efficacy of CERA for correcting anemia in Korean patients on dialysis. Patients (> or =18 yr) who were not receiving any ESAs for more than 8 weeks were randomly assigned to either intravenous CERA once every 2 weeks (n=39) or epoetin beta thrice-weekly (n=41) during a 24-week correction phase. Hemoglobin (Hb) response was defined as increase of Hb by at least 1 g/dL and Hb> or =11 g/dL without red blood cell (RBC) transfusion. Median dialysis duration was 1.7 (0.3-20.8) and 1.6 (0.4-13.8) yr in CERA and epoetin beta group, respectively. Hemoglobin response rate of CERA was 79.5% (95% confidence interval [CI], 63.5-90.7). As the lower limit of 95% CI was higher than pre-specified 60% response rate, it can be concluded that CERA corrected anemia (P<0.05). Hb response rate of epoetin beta was 87.8% (95% CI, 73.8-95.9) (P=0.37). Median time to response was 12 weeks in CERA and 10.3 weeks in epoetin beta (P=0.03). It is suggested that once every 2 weeks administration of CERA is effective for correcting anemia in Korean patients on long-term hemodialysis with longer time-to-response than thrice weekly epoetin beta. (ClinicalTrials.gov registry No. NCT00546481)
Keyword
MeSH Terms
-
Anemia/*drug therapy
Erythropoietin/*therapeutic use
Female
Hemoglobins/analysis
Humans
Male
Middle Aged
Polyethylene Glycols/*therapeutic use
Quality of Life
Recombinant Proteins/therapeutic use
Renal Dialysis
Renal Insufficiency, Chronic/*drug therapy
Republic of Korea
Hemoglobins
Polyethylene Glycols
Recombinant Proteins
Erythropoietin
Figure
Reference
-
1. Henry DH, Bowers P, Romano MT, Provenzano R. Epoetin alfa: clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004; 164:262–276.2. Curran MP, McCormack PL. Methoxy polyethylene glycol-epoetin beta: a review of its use in the management of anaemia associated with chronic kidney disease. Drugs. 2008; 68:1139–1156.3. Macdougall IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, Dougherty FC, Reigner B. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006; 1:1211–1215.4. Klinger M, Arias M, Vargemezis V, Besarab A, Sulowicz W, Gerntholtz T, Ciechanowski K, Dougherty FC, Beyer U. Efficacy of intravenous methoxy polyethylene glycol-epoetin beta administered every 2 weeks compared with epoetin administered 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomized trial. Am J Kidney Dis. 2007; 50:989–1000.5. Macdougall IC, Walker R, Provenzano R, de Alvaro F, Locay HR, Nader PC, Locatelli F, Dougherty FC, Beyer U. ARCTOS Study Investigators. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin J Am Soc Nephrol. 2008; 3:337–347.6. Levin NW, Fishbane S, Cañedo FV, Zeig S, Nassar GM, Moran JE, Villa G, Beyer U, Oguey D. MAXIMA Study Investigators. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA). Lancet. 2007; 370:1415–1421.7. Sulowicz W, Locatelli F, Ryckelynck JP, Balla J, Csiky B, Harris K, Ehrhard P, Beyer U. PROTOS Study Investigators. Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007; 2:637–646.8. Canaud B, Mingardi G, Braun J, Aljama P, Kerr PG, Locatelli F, Villa G, Van Vlem B, McMahon AW, Kerloëguen C, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transplant. 2008; 23:3654–3661.9. Spinowitz B, Coyne DW, Lok CE, Fraticelli M, Azer M, Dalal S, Villa G, Rosansky S, Adamis H, Beyer U. C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol. 2008; 28:280–289.10. Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009; 24:2502–2510.11. Penne EL, van der Weerd NC, Grooteman MP, Mazairac AH, van den Dorpel MA, Nubé MJ, Bots ML, Lévesque R, ter Wee PM, Blankestijn PJ. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011; 6:281–289.12. Locatelli F, Reigner B. C.E.R.A.: pharmacodynamics, pharmacokinetics and efficacy in patients with chronic kidney disease. Expert Opin Investig Drugs. 2007; 16:1649–1661.13. Fishbane S, Pannier A, Liogier X, Jordan P, Dougherty FC, Reigner B. Pharmacokinetic and pharmacodynamic properties of methoxy polyethylene glycol-epoetin beta are unaffected by the site of subcutaneous administration. J Clin Pharmacol. 2007; 47:1390–1397.14. Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004; 19:ii1–ii47.15. KDOQI. National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006; 47:S11–S145.16. Fliser D, Kleophas W, Dellanna F, Winkler RE, Backs W, Kraatz U, Fassbinder W, Wizemann V, Strack G. Evaluation of maintenance of stable haemoglobin levels in haemodialysis patients converting from epoetin or darbepoetin to monthly intravenous C.E.R.A.: the MIRACEL study. Curr Med Res Opin. 2010; 26:1083–1089.17. Weinreich T, Leistikow F, Hartmann HG, Vollgraf G, Dellanna F. SESAM Study Group. Monthly continuous erythropoietin receptor activator treatment maintains stable hemoglobin levels in routine clinical management of hemodialysis patients. Hemodial Int. 2012; 16:11–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the serum erythropoietin levels in neonates
- Clinical Characteristics of Poor Responders to Erythropoietin among ESRD Patients Undergoing Hemodialysis
- Efficacy and cost-effectiveness of darbepoetin alfa once every 4 weeks versus continuous erythropoietin receptor activator once every 4 weeks for anemia correction in patients with chronic kidney disease not on dialysis
- Clinical evaluation of hemochromatosis with end-stage renal disease
- Efficacy of Protocol-based Erythropoietin Administration in Chronic Hemodialysis Patients