J Korean Med Sci.
2013 Sep;28(9):1351-1355. 10.3346/jkms.2013.28.9.1351.
Influence of Body Mass Index on the Growth Hormone Response to Provocative Testing in Short Children without Growth Hormone Deficiency
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, SMG-SNU Boramae Medical Center, Seoul, Korea. gnoygnoes@hanmail.net
- KMID: 1793052
- DOI: http://doi.org/10.3346/jkms.2013.28.9.1351
Abstract
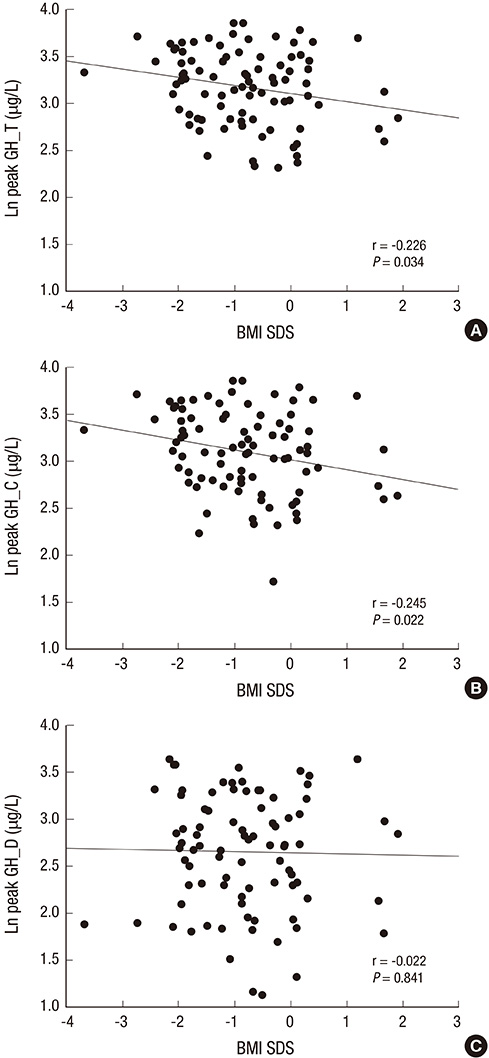
- Obesity and its related factors are known to suppress the secretion of growth hormone (GH). We aimed to evaluate the influence of body mass index (BMI) on the peak GH response to provocative testing in short children without GH deficiency. We conducted a retrospective review of medical records of 88 children (2-15 yr old) whose height was less than 3 percentile for one's age and sex, with normal results (peak GH level > 10 ng/mL) of GH provocative testing with clonidine and dopamine. Peak stimulated GH level, height, weight, pubertal status and serum IGF-1 level were measured. Univariate analysis showed that the BMI standard deviation score (SDS) correlated negatively with the natural log (ln) of the peak stimulated GH level (ln peak GH). BMI SDS did not correlate significantly with sex, age, pubertal status, or ln IGF-1 level. BMI SDS correlated negatively with ln peak GH level induced by clonidine but not by dopamine. In stepwise multivariate regression analysis, BMI SDS was the only significant predictor of ln peak GH level in the combination of tests and the clonidine test, but not in the dopamine test. In children without GH deficiency, BMI SDS correlates negatively with the peak GH level. BMI SDS should be included in the analysis of the results of GH provocation tests, especially tests with clonidine.
Keyword
MeSH Terms
-
Adolescent
Body Height
*Body Mass Index
Body Weight
Child
Child, Preschool
Clonidine/therapeutic use
Dopamine/therapeutic use
Dwarfism/drug therapy
Female
Human Growth Hormone/*analysis
Humans
Insulin-Like Growth Factor I/analysis
Male
Regression Analysis
Retrospective Studies
Clonidine
Dopamine
Human Growth Hormone
Insulin-Like Growth Factor I
Figure
Cited by 2 articles
-
Growth Hormone Responses to Provocative Tests in Children with Short Stature
Noorisaem Rhee, Ka Young Oh, Eun Mi Yang, Chan Jong Kim
Chonnam Med J. 2015;51(1):33-38. doi: 10.4068/cmj.2015.51.1.33.Effect of body mass index on peak growth hormone level after growth hormone stimulation test in children with short stature
Na Yeong Lee, Sung Eun Kim, Seulki Kim, Moon Bae Ahn, Shin Hee Kim, Won Kyoung Cho, Kyoung Soon Cho, Min Ho Jung, Byung-Kyu Suh
Ann Pediatr Endocrinol Metab. 2021;26(3):192-198. doi: 10.6065/apem.2040246.123.
Reference
-
1. Cacciari E, Tassoni P, Cicognani A, Pirazzoli P, Salardi S, Balsamo A, Cassio A, Zucchini S, Colli C, Tassinari D, et al. Value and limits of pharmacological and physiological tests to diagnose growth hormone (GH) deficiency and predict therapy response: first and second retesting during replacement therapy of patients defined as GH deficient. J Clin Endocrinol Metab. 1994; 79:1663–1669.2. Maghnie M, Strigazzi C, Tinelli C, Autelli M, Cisternino M, Loche S, Severi F. Growth hormone (GH) deficiency (GHD) of childhood onset: reassessment of GH status and evaluation of the predictive criteria for permanent GHD in young adults. J Clin Endocrinol Metab. 1999; 84:1324–1328.3. Zadik Z, Chalew SA, Gilula Z, Kowarski AA. Reproducibility of growth hormone testing procedures: a comparison between 24-hour integrated concentration and pharmacological stimulation. J Clin Endocrinol Metab. 1990; 71:1127–1130.4. Marin G, Domené HM, Barnes KM, Blackwell BJ, Cassorla FG, Cutler GB Jr. The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J Clin Endocrinol Metab. 1994; 79:537–541.5. Rose SR, Municchi G, Barnes KM, Kamp GA, Uriarte MM, Ross JL, Cassorla F, Cutler GB Jr. Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J Clin Endocrinol Metab. 1991; 73:428–435.6. Devesa J, Lima L, Lois N, Fraga C, Lechuga MJ, Arce V, Tresguerres JA. Reasons for the variability in growth hormone (GH) responses to GHRH challenge: the endogenous hypothalamic-somatotroph rhythm (HSR). Clin Endocrinol (Oxf). 1989; 30:367–377.7. Maghnie M, Valtorta A, Moretta A, Larizza D, Preti P, Palladini G, Calcante S, Severi F. Diagnosing growth hormone deficiency: the value of short-term hypocaloric diet. J Clin Endocrinol Metab. 1993; 77:1372–1378.8. Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA. Impaired growth hormone responses to growth hormone-releasing factor in obesity: a pituitary defect reversed with weight reduction. N Engl J Med. 1984; 311:1403–1407.9. Stanley TL, Levitsky LL, Grinspoon SK, Misra M. Effect of body mass index on peak growth hormone response to provocative testing in children with short stature. J Clin Endocrinol Metab. 2009; 94:4875–4881.10. Loche S, Guzzetti C, Pilia S, Ibba A, Civolani P, Porcu M, Minerba L, Casini MR. Effect of body mass index on the growth hormone response to clonidine stimulation testing in children with short stature. Clin Endocrinol (Oxf). 2011; 74:726–731.11. Lee HS, Hwang JS. Influence of body mass index on growth hormone responses to classic provocative tests in children with short stature. Neuroendocrinology. 2011; 93:259–264.12. Greulich WW, Pyle SI. Radiologic atlas of skeletal development of the hand and wrist. 2nd ed. Standford: University Press;1959.13. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, et al. 2007 Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51:1–25.14. Loche S, Cambiaso P, Carta D, Setzu S, Imbimbo BP, Borrelli P, Pintor C, Cappa M. The growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, in short normal and obese children and in hypopituitary subjects. J Clin Endocrinol Metab. 1995; 80:674–678.15. Bonert VS, Elashoff JD, Barnett P, Melmed S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab. 2004; 89:3397–3401.16. Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab. 1991; 72:51–59.17. Pincelli AI, Rigamonti AE, Scacchi M, Cella SG, Cappa M, Cavagnini F, Müller EE. Somatostatin infusion withdrawal: studies in the acute and recovery phase of anorexia nervosa, and in obesity. Eur J Endocrinol. 2003; 148:237–243.18. Volta C, Bernasconi S, Iughetti L, Ghizzoni L, Rossi M, Costa M, Cozzini A. Growth hormone response to growth hormone-releasing hormone (GHRH), insulin, clonidine and arginine after GHRH pretreatment in obese children: evidence of somatostatin increase? Eur J Endocrinol. 1995; 132:716–721.19. Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab. 2008; 295:E385–E392.20. Scacchi M, Orsini F, Cattaneo A, Grasso A, Filippini B, Pecori Giraldi F, Fatti LM, Moro M, Cavagnini F. The diagnosis of GH deficiency in obese patients: a reappraisal with GHRH plus arginine testing after pharmacological blockade of lipolysis. Eur J Endocrinol. 2010; 163:201–206.21. Alvarez CV, Mallo F, Burguera B, Cacicedo L, Dieguez C, Casanueva FF. Evidence for a direct pituitary inhibition by free fatty acids of in vivo growth hormone responses to growth hormone-releasing hormone in the rat. Neuroendocrinology. 1991; 53:185–189.22. Casanueva FF, Villanueva L, Dieguez C, Diaz Y, Cabranes JA, Szoke B, Scanlon MF, Schally AV, Fernandez-Cruz A. Free fatty acids block growth hormone (GH) releasing hormone-stimulated GH secretion in man directly at the pituitary. J Clin Endocrinol Metab. 1987; 65:634–642.23. Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, Grottoli S, Maccario M, Colao A, Lombardi G, et al. The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol. 2005; 153:257–254.24. Ghigo E, Bellone J, Aimaretti G, Bellone S, Loche S, Cappa M, Bartolotta E, Dammacco F, Camanni F. Reliability of provocative tests to assess growth hormone secretory status: study in 472 normally growing children. J Clin Endocrinol Metab. 1996; 81:3323–3327.25. Gil-Ad I, Laron Z, Koch Y. Effect of acute and chronic administration of clonidine on hypothalamic content of growth hormone-releasing hormone and somatostatin in the rat. J Endocrinol. 1991; 13:381–385.26. Hanew K, Utsumi A. The role of endogenous GHRH in arginine-, insulin-, clonidine- and l-dopa-induced GH release in normal subjects. Eur J Endocrinol. 2002; 146:197–202.27. García-Tornadu I, Risso G, Perez-Millan MI, Noain D, Diaz-Torga G, Low MJ, Rubinstein M, Becu-Villalobos D. Neurotransmitter modulation of the GHRH-GH axis. Front Horm Res. 2010; 38:59–69.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical effects of yeast derived recombinant methionyl growth hormone in growth hormone deficiency
- Growth hormone deficiency in adult
- Diagnostic value of insulin-like growth factor-I in short stature
- Effect of yeast-derived methionyl recombinant growth hormone on growth hormone deficient dwarf
- Comparison of Growth Hormone Secretory Ability between Insulin, Propranolol-levodopa, and Clonidine in Growth Hormone Deficiency Patients