Korean J Ophthalmol.
2014 Feb;28(1):39-48. 10.3341/kjo.2014.28.1.39.
Diurnal Intraocular Pressure with Bimatoprost/Timolol Fixed Combination versus Latanoprost/Timolol Fixed Combination in Healthy Subjects
- Affiliations
-
- 1Department of Ophthalmology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. kjoonmo@dreamwiz.com
- 2Department of Ophthalmology, Institute of Vision Research, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1792093
- DOI: http://doi.org/10.3341/kjo.2014.28.1.39
Abstract
- PURPOSE
To evaluate the effects of a bimatoprost/timolol fixed combination (BTFC) and a latanoprost/timolol fixed combination (LTFC) on diurnal intraocular pressure (IOP) and anterior ocular parameters in healthy subjects.
METHODS
We enrolled 58 healthy subjects in this prospective clinical study. Thirty subjects were treated with BTFC and 28 subjects were treated with LTFC. IOP was measured every 2 hours except from 01:00 and 05:00. Axial length, corneal curvature, and anterior chamber depth were obtained using the IOL master at baseline and 24 hours later. Adverse events were assessed by patient interview and by slit lamp examination.
RESULTS
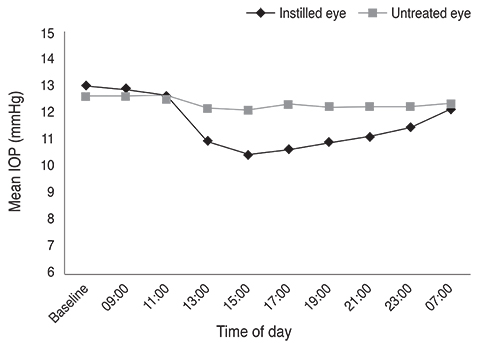
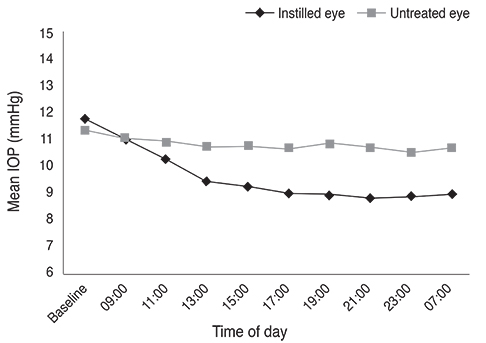
The largest difference in IOP between treated and untreated eyes 8 hours after instillation was 1.67 mmHg in the BTFC group (p < 0.001). The largest difference in IOP between treated and untreated eyes 10 hours after instillation was 1.93 mmHg in the LTFC group (p < 0.001). For anterior ocular parameters such as axial length, corneal curvature, anterior chamber depth at baseline and 24 hours after instillation, there were no significant differences between the baseline and 24-hour values in either the BTFC or LTFC group. The most frequently occurring adverse event was conjunctival hyperemia, which was found in 33.3% (n = 10) of the BTFC group and 25.0% (n = 7) of the LTFC group (p = 0.486).
CONCLUSIONS
BTFC and LTFC provided a significant reduction in IOP from baseline without changing any anterior ocular parameters. Our results provide a reference for monocular trials to assess the effect of eye drops in a clinical condition.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Amides/*administration & dosage
Antihypertensive Agents/administration & dosage
Circadian Rhythm/*physiology
Cloprostenol/administration & dosage/*analogs & derivatives
Dose-Response Relationship, Drug
Drug Therapy, Combination
Female
Follow-Up Studies
Glaucoma, Open-Angle/drug therapy/*physiopathology
Healthy Volunteers
Humans
Intraocular Pressure/drug effects/*physiology
Male
Middle Aged
Ophthalmic Solutions
Prospective Studies
Prostaglandins F, Synthetic/*administration & dosage
Timolol/*administration & dosage
Tonometry, Ocular
Treatment Outcome
Amides
Antihypertensive Agents
Cloprostenol
Ophthalmic Solutions
Prostaglandins F, Synthetic
Timolol
Figure
Reference
-
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90:262–267.2. Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998; 116:653–658.3. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120:1268–1279.4. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–713.5. Fremont AM, Lee PP, Mangione CM, et al. Patterns of care for open-angle glaucoma in managed care. Arch Ophthalmol. 2003; 121:777–783.6. Diestelhorst M, Almegard B. Comparison of two fixed combinations of latanoprost and timolol in open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1998; 236:577–581.7. Larsson LI. Effect on intraocular pressure during 24 hours after repeated administration of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertension. J Glaucoma. 2001; 10:109–114.8. Larsson LI. The effect on diurnal intraocular pressure of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertension. Acta Ophthalmol Scand. 2001; 79:125–128.9. Higginbotham EJ, Feldman R, Stiles M, et al. Latanoprost and timolol combination therapy vs monotherapy: one-year randomized trial. Arch Ophthalmol. 2002; 120:915–922.10. Pfeiffer N. European Latanoprost Fixed Combination Study Group. A comparison of the fixed combination of latanoprost and timolol with its individual components. Graefes Arch Clin Exp Ophthalmol. 2002; 240:893–899.11. Diestelhorst M, Larsson LI. European Latanoprost Fixed Combination Study Group. A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension. Br J Ophthalmol. 2004; 88:199–203.12. Shin DH, Feldman RM, Sheu WP, et al. Efficacy and safety of the fixed combinations latanoprost/timolol versus dorzolamide/timolol in patients with elevated intraocular pressure. Ophthalmology. 2004; 111:276–282.13. Konstas AG, Lake S, Economou AI, et al. 24-Hour control with a latanoprost-timolol fixed combination vs timolol alone. Arch Ophthalmol. 2006; 124:1553–1557.14. Martinez A, Sanchez M. Bimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patients. Eye (Lond). 2009; 23:810–818.15. Lewis RA, Gross RL, Sall KN, et al. The safety and efficacy of bimatoprost/timolol fixed combination: a 1-year double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2010; 19:424–426.16. Brandt JD, Cantor LB, Katz LJ, et al. Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2008; 17:211–216.17. Konstas AG, Katsimbris JM, Lallos N, et al. Latanoprost 0.005% versus bimatoprost 0.03% in primary open-angle glaucoma patients. Ophthalmology. 2005; 112:262–266.18. Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005; 112:863–868.19. Hasegawa K, Ishida K, Sawada A, et al. Diurnal variation of intraocular pressure in suspected normal-tension glaucoma. Jpn J Ophthalmol. 2006; 50:449–454.20. Tajunisah I, Reddy SC, Fathilah J. Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1851–1857.21. Sit AJ, Liu JH, Weinreb RN. Asymmetry of right versus left intraocular pressures over 24 hours in glaucoma patients. Ophthalmology. 2006; 113:425–430.22. Liu JH, Sit AJ, Weinreb RN. Variation of 24-hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology. 2005; 112:1670–1675.23. Lee JY, Hwang YH, Kim YY. The efficacy of a monocular drug trial in normal-tension glaucoma. Korean J Ophthalmol. 2012; 26:26–31.24. Realini T, Fechtner RD, Atreides SP, Gollance S. The uniocular drug trial and second-eye response to glaucoma medications. Ophthalmology. 2004; 111:421–426.25. Dayanir V, Cakmak H, Berkit I. The one-eye trial and fellow eye response to prostaglandin analogues. Clin Experiment Ophthalmol. 2008; 36:136–141.26. Chaudhary O, Adelman RA, Shields MB. Predicting response to glaucoma therapy in one eye based on response in the fellow eye: the monocular trial. Arch Ophthalmol. 2008; 126:1216–1220.27. Takahashi M, Higashide T, Sakurai M, Sugiyama K. Discrepancy of the intraocular pressure response between fellow eyes in one-eye trials versus bilateral treatment: verification with normal subjects. J Glaucoma. 2008; 17:169–174.28. Noecker RS, Dirks MS, Choplin NT, et al. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003; 135:55–63.29. Orzalesi N, Rossetti L, Bottoli A, Fogagnolo P. Comparison of the effects of latanoprost, travoprost, and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology. 2006; 113:239–246.30. Parrish RK, Palmberg P, Sheu WP. XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003; 135:688–703.31. Woodward DF, Krauss AH, Chen J, et al. Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024). J Pharmacol Exp Ther. 2003; 305:772–785.32. Sharif NA, Kelly CR, Crider JY, et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003; 19:501–515.33. Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004; 78:767–776.34. Cho SW, Kim JM, Park KH, Choi CY. Effects of brimonidine 0.2%-timolol 0.5% fixed-combination therapy for glaucoma. Jpn J Ophthalmol. 2010; 54:407–413.35. Konstas AG, Boboridis K, Tzetzi D, et al. Twenty-four-hour control with latanoprost-timolol-fixed combination therapy vs latanoprost therapy. Arch Ophthalmol. 2005; 123:898–902.36. Simsek S, Yulek F, Cakmak HB, Midillioglu IK. Long-term effects of prostaglandin analogues on the anterior chamber depth of patients with primary open-angle glaucoma. Cutan Ocul Toxicol. 2009; 28:125–128.37. Gutierrez-Ortiz C, Teus MA, Bolivar G. Short-term effects of latanoprost on anterior chamber depth in patients with glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2006; 47:4856–4859.38. Martinez A, Sanchez M. A comparison of the safety and intraocular pressure lowering of bimatoprost/timolol fixed combination versus latanoprost/timolol fixed combination in patients with open-angle glaucoma. Curr Med Res Opin. 2007; 23:1025–1032.39. Chen J, Dinh T, Woodward DF, et al. Bimatoprost: mechanism of ocular surface hyperemia associated with topical therapy. Cardiovasc Drug Rev. 2005; 23:231–246.40. Russo A, Riva I, Pizzolante T, et al. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review. Clin Ophthalmol. 2008; 2:897–905.41. Feldman RM. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2003; 19:23–35.42. Bhorade AM. The monocular trial controversy: a critical review. Curr Opin Ophthalmol. 2009; 20:104–109.43. Drance SM. The uniocular therapeutic trial in the management of elevated intraocular pressure. Surv Ophthalmol. 1980; 25:203–205.44. Piltz J, Gross R, Shin DH, et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am J Ophthalmol. 2000; 130:441–453.45. Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003; 44:1586–1590.46. Wax MB, Camras CB, Fiscella RG, et al. Emerging perspectives in glaucoma: optimizing 24-hour control of intraocular pressure. Am J Ophthalmol. 2002; 133:Suppl. S1–S10.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Add-on Effect of Prostaglandin Analogues in Dorzolamide/Timolol Fixed Combination Treated Primary Open Angle Glaucoma Patients
- The Effect of a Fixed Combination of 0.0015% Tafluprost-0.5% Timolol in Normal Tension Glaucoma Patients
- Efficacy and Safety of a Fixed Combination of Bimatoprost (0.03% w/v) and Timolol (0.5% w/v) for Patients with Open-angle Glaucoma
- Long-term Results of Selective Laser Trabeculoplasty versus Latanoprost or Dorzolamide/Timolol Fixed Combination
- Comparison of Dorzolamide-Timolol Fixed Combination and Latanoprost, Effects on Intraocular Pressure and Ocular Pulse Amplitude