Blood Res.
2015 Mar;50(1):19-25. 10.5045/br.2015.50.1.19.
Efficacy and safety of eltrombopag in adult refractory immune thrombocytopenia
- Affiliations
-
- 1Department of Hematology, Chonnam National University Hwasun Hospital, Hwasun, Korea. yeokim@jnu.ac.kr
- KMID: 1787908
- DOI: http://doi.org/10.5045/br.2015.50.1.19
Abstract
- BACKGROUND
Eltrombopag is a thrombopoietin receptor agonist with excellent treatment outcomes in immune thrombocytopenia (ITP). Here, we analyzed the dose of eltrombopag required to achieve and maintain safe platelet counts in Korean ITP patients.
METHODS
Adult refractory ITP patients (<30,000 platelets/microL) were enrolled. Eltrombopag doses were increased to achieve a target platelet count (> or =50,000 cells/microL). After achieving the target platelet count, the dose of concomitant ITP medications and eltrombopag was reduced to identify the lowest effective dose required to maintain the platelet count.
RESULTS
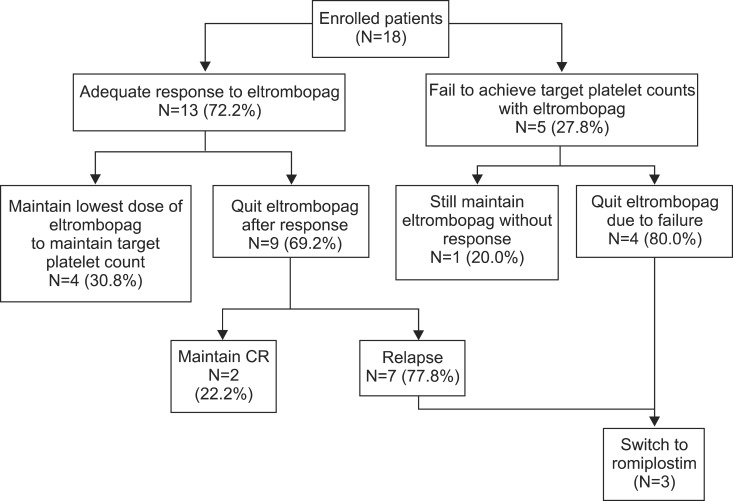
Among 18 patients, 66.7% achieved complete response, 5.6% achieved platelet counts between 50,000 and 100,000 cells/microL, and 27.8% failed to achieve the target platelet count. The median ITP duration was significantly shorter in patients who achieved the target platelet count. The initial dose required to achieve the target platelet count was 25 mg/d. The adjusted maintenance doses were 25 mg twice per week or 25 mg/d. After discontinuation, 83.3% relapsed, and the median relapse-free survival was 15 days. Two relapsed and 1 failed patient switched to romiplostim. The response to romiplostim was similar to eltrombopag. During eltrombopag treatment, 38.9% showed hepatobiliary laboratory anomalies. Among 9 follow-up bone marrow examinations, 1 revealed fibrosis after 1 year of treatment.
CONCLUSION
Eltrombopag was well tolerated with excellent treatment outcomes in refractory adult ITP patients. Low-dose eltrombopag effectively maintained the target platelet count. However, some patients required longer or higher-dose treatment to maintain the target platelet count, especially in heavily pretreated or longer ITP cases.
MeSH Terms
Figure
Cited by 3 articles
-
Eltrombopag: a new treatment option for chronic refractory adult immune thrombocytopenia
Rojin Park
Blood Res. 2015;50(1):1-2. doi: 10.5045/br.2015.50.1.1.Steroid-refractory immune thrombocytopenia in the era of the new thrombomimetic drugs: is there still a role for rituximab?
Massimiliano Palombi, Laura Scaramucci, Marco Giovannini, Malgorzata Monika Trawinska, Pasquale Niscola, Paolo de Fabritiis
Blood Res. 2016;51(1):66-66. doi: 10.5045/br.2016.51.1.66.Delayed treatment-free response after romiplostim discontinuation in pediatric chronic immune thrombocytopenia
Hyun Ji Lim, Young Tae Lim, Jeong Ok Hah, Jae Min Lee
Yeungnam Univ J Med. 2020;38(2):165-168. doi: 10.12701/yujm.2020.00493.
Reference
-
1. Cheng G. Eltrombopag, a thrombopoietin-receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol. 2012; 3:155–164. PMID: 23556122.2. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011; 377:393–402. PMID: 20739054.
Article3. Saleh MN, Bussel JB, Cheng G, et al. EXTEND Study Group. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013; 121:537–545. PMID: 23169778.
Article4. Shida Y, Takahashi N, Nohda S, Hirama T. Pharmacokinetics and pharmacodynamics of eltrombopag in healthy Japanese males. Jpn J Clin Pharmacol Ther. 2011; 42:11–20.
Article5. Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011; 51:842–856. PMID: 20663993.
Article6. Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012; 10:799–806. PMID: 22409309.
Article7. Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Semin Hematol. 2013; 50(Suppl 1):S18–S21. PMID: 23664510.
Article8. Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013; 53:2807–2812. PMID: 23451917.
Article9. Aoki T, Harada Y, Matsubara E, et al. Thrombopoietin receptor agonists in refractory immune thrombocytopenia: differential responses to eltrombopag and romiplostim: a case report and possible explanations. J Clin Pharm Ther. 2012; 37:729–732. PMID: 22583038.
Article10. Tsukamoto S, Nakaseko C, Takeuchi M, et al. Safety and efficacy of romiplostim in patients with eltrombopag-resistant or -intolerant immune thrombocytopenia. Br J Haematol. 2013; 163:286–289. PMID: 23862773.
Article11. Sartori R, Candiotto L, Ruggeri M, Tagariello G. Immune thrombocytopenia successfully treated with eltrombopag following multiple therapies including romiplostim. Blood Transfus. 2014; 12(Suppl 1):s151–s152. PMID: 24120607.12. Polverelli N, Palandri F, Iacobucci I, Catani L, Martinelli G, Vianelli N. Absence of bi-directional cross-resistance of thrombopoietin receptor agonists in chronic refractory immune thrombocytopenia: possible role of MPL polymorphisms. Br J Haematol. 2013; 161:142–144. PMID: 23278214.13. Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013; 121:2596–2606. PMID: 23361904.
Article14. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009; 113:2386–2393. PMID: 19005182.
Article15. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117:4190–4207. PMID: 21325604.
Article16. Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005; 90:1128–1132. PMID: 16079113.17. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009; 373:641–648. PMID: 19231632.
Article18. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008; 371:395–403. PMID: 18242413.
Article19. Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013; 161:411–423. PMID: 23432528.
Article20. Leven E, Miller A, Boulad N, Haider A, Bussel JB. Successful discontinuation of eltrombopag treatment in patients with chronic ITP. Blood. 2012; 120(ASH Annual Meeting):abst 1085.
Article21. Vlachaki E, Sousos N, Perifanis V, et al. Is there a role for low-dose eltrombopag as maintenance therapy in the treatment of immune thrombocytopenia? Acta Haematol. 2015; 133:78–82. PMID: 25170628.
Article22. Khellaf M, Viallard JF, Hamidou M, et al. A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica. 2013; 98:881–887. PMID: 23445876.
Article23. Scaramucci L, Giovannini M, Niscola P, Tendas A, Perrotti A, De Fabritiis P. Reciprocal absence of cross-resistance between eltrombopag and romiplostim in two patients with refractory immune thrombocytopenic purpura. Blood Transfus. 2014; 12:605–607. PMID: 25351191.24. Piccin A, Amaddii G, Natolino F, Billio A, Cortelazzo S. Idiopathic thrombocytopenic purpura resistant to eltrombopag, but cured with romiplostim. Blood Transfus. 2014; 12(Suppl 1):s149–s150. PMID: 23736912.25. D'Arena G, Guariglia R, Mansueto G, et al. No cross-resistance after sequential use of romiplostim and eltrombopag in chronic immune thrombocytopenic purpura. Blood. 2013; 121:1240–1242. PMID: 23411736.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eltrombopag: a new treatment option for chronic refractory adult immune thrombocytopenia
- Dapsone therapy for refractory immune thrombocytopenia patients: a case series
- Application of vincristine-loaded platelet therapy in three dogs with refractory immune-mediated thrombocytopenia
- Romiplostim plus danazol as salvage treatment for eltrombopag refractory immune thrombocytopenia: a retrospective pilot study
- Diagnostic Approach of Childhood Immune Thrombocytopenia