J Korean Med Assoc.
2012 Sep;55(9):827-834. 10.5124/jkma.2012.55.9.827.
Current status of pharmaceutical safety management in Korea
- Affiliations
-
- 1Pharmaceutical Safety Information Team, Pharmaceutical Safety Bureau, Korea Food and Drug Administration, Cheongwon, Korea. donwoong@korea.kr
- KMID: 1787245
- DOI: http://doi.org/10.5124/jkma.2012.55.9.827
Abstract
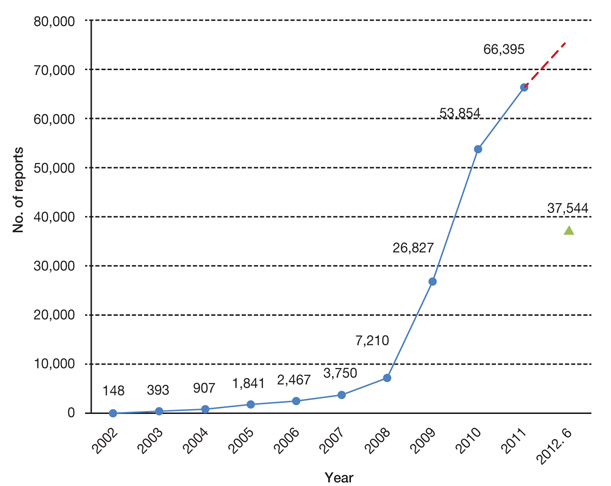
- The reinforcement of regulation on of post-market safety management including adverse drug reactions (ADRs) has received significant emphasiszed significantly over the last several years in Korea. Not only has there been an increase in the number of spontaneous reports on ADRs, but an amendment of to the pharmaceutical law has been passed and notifications have noticeably been accelerated noticeably. However, compared with advanced countries, the efficiency of the system and people's satisfaction withon post-market safety management was has been as low as ever. This article focuses on the state of the regulations with regard to reporting of ADRs information. In addition, the status and kinds of drug utilization review informations offered by the Korea Food and Drug Administration were are illustrated in detail.
MeSH Terms
Figure
Reference
-
1. Food and Drug Administration Amendments Act of 2007, Pub. L. No. 110-85 (Sep 27, 2007).2. European Commission. Volume 9A of the rules governing medicinal products in the European Union: guidelines on pharmacovigilance for medicinal products for human use. 2008. Bruxelles: European Commission.3. Levinthal CF. Drugs, behavior, and modern society. 2005. 4th ed. Boston: Pearson Education.4. The standard of toxicity test of pharmaceutical products: notification of Korea Food and Drug Administration under the title of 2009-116. (amendment in Aug 24, 2009).5. Korea Food and Drug Administration. Guideline for non-clinical trial for performance of clinical trial and marketing authorization of pharmaceutical products. 2012. Cheongwon: Korea Food and Drug Administration.6. The regulation about post-market review of new drug, etc.: notification of Korea Food and Drug Administration under the title of 2011-60. (amendment in Oct 10, 2011).7. The regulation about implementation of drug re-evaluation: notification of Korea Food and Drug Administration under the title of 2012-4. (amendment in Feb 23, 2012).8. The regulation of safety information management of drug, etc.: notification of Korea Food and Drug Administration under the title of 2012-18. (amendment in May 15, 2012).9. World Health Organization. Drug and therapeutics committees: a practical guide. 2003. Geneva: World Health Organization.10. The regulation of adverse drug reaction committee: regulation of Korea Food and Drug Administration under the title of 2012-239.11. US Pharmacopeia Drug Utilization Review Advisory Panel. Drug utilization review: mechanisms to improve its effectiveness and broaden its scope. J Am Pharm Assoc (Wash). 2000. 40:538–545.12. Choi KE, Oh OH. Drug information development for drug utilization review system in USA. J Korean Acad Manag Care Pharm. 2005. 1:28–32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Introducing Patient Safety Activities in Canada: Focused on Medication Incidents and Preventions
- Current Status of Post-marketing Safety Management in United States, Europe and Japan: Risk Management Plan
- The Current Status of the Organizations Dedicated to Post-marketing Safety Management of Pharmaceuticals
- Status of Domestic and International Recommendations for Protection Design and Evaluation of Medical Linear Accelerator Facilities
- A survey for Management of Drug Safety Evaluation System for Investigational Product