J Korean Med Sci.
2004 Feb;19(1):42-50. 10.3346/jkms.2004.19.1.42.
Effects of pH on Vascular Tone in Rabbit Basilar Arteries
- Affiliations
-
- 1Department of Physiology, Chungbuk National University College of Medicine, Cheongju, Korea. kimkw@plaza.snu.ac.kr
- 2Department of Physiology and Biophysics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1785692
- DOI: http://doi.org/10.3346/jkms.2004.19.1.42
Abstract
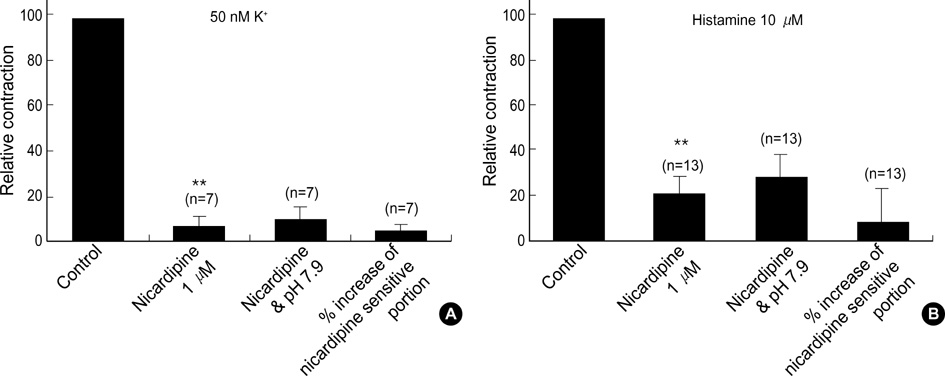
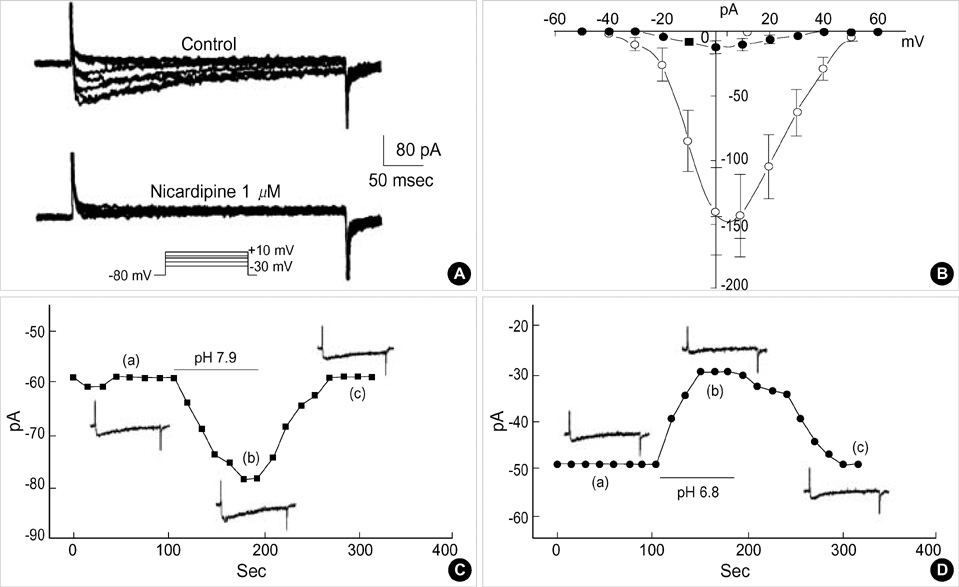
- Effects of pH on vascular tone and L-type Ca2+ channels were investigated using Mulvany myograph and voltage-clamp technique in rabbit basilar arteries. In rabbitbasilar arteries, high K+ produced tonic contractions by 11+/-0.6 mN (mean+/-S.E., n=19). When extracellular pH (pHo) was changed from control 7.4 to 7.9 ([alkalosis]o), K+-induced contraction was increased to 128+/-2.1% of the control (n=13). However, K+-induced contraction was decreased to 73+/-1.3% of the control at pHo 6.8 ([acidosis]o, n=4). Histamine (10 micrometer) also produced tonic contraction by 11+/-0.6 mN (n=17), which was blocked by post-application of nicardipine (1 micrometer). [alkalosis]o and [acidosis]o increased or decreased histamine-induced contraction to 134+/-5.7% and 27+/-7.6% of the control (n=4, 6). Since high K+- and histamine-induced tonic contractions were affected by nicardipine and pHo, the effect of pHo on voltage-dependent L-type Ca2+ channel (VDCCL) was studied. VDCCL was modulated by pHo: the peak value of Ca2+ channel current (IBa) at a holding of 0 mV decreased in [acidosis]o by 41+/-8.8%, whereas that increased in [alkalosis]o by 35+/-2.1% (n=3). These results suggested that the external pH regulates vascular tone partly via the modulation of VDCC in rabbit basilar arteries.
Keyword
MeSH Terms
-
Animals
Arteries/*pathology
Basilar Artery/*pathology
Calcium/metabolism
Calcium Channels/chemistry
Electrophysiology
Histamine/chemistry/metabolism
Hydrogen-Ion Concentration
Muscle Cells/cytology
Muscle Contraction
Muscle, Smooth/*pathology
Patch-Clamp Techniques
Potassium/chemistry/metabolism
Rabbits
Stress, Mechanical
Time Factors
Figure
Reference
-
1. Tian R, Vogel P, Lassen NA, Mulvany MJ, Andreasen F, Aalkjaer C. Role of extracellular and intracellular acidosis for hypercapnia-induced inhibition of tension of isolated rat cerebral arteries. Circ Res. 1995. 76:269–275.
Article2. Kontos HA. Regulation of the cerebral circulation. Annu Rev Physiol. 1981. 43:397–407.
Article3. Toda N, Hatano Y, Mori K. Mechanisms underlying response to hypercapnia and bicarbonate of isolated dog cerebral arteries. Am J Physiol. 1989. 257:H141–H146.
Article4. Klöckner U, Isenberg G. Intracellular pH modulates the avalability of vascular L-type Ca2+ channels. J Gen Physiol. 1994. 103:647–663.5. Klöckner U, Isenberg G. Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J Gen Physiol. 1994. 103:665–678.
Article6. Smith JB, Dwyer SD, Smith L. Lowering extracellular pH evokes inositol polyphosphate formation and calcium mobilization. J Biol Chem. 1989. 264:8723–8728.
Article7. Ishizaka H, Kuo L. Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circ Res. 1996. 78:50–57.
Article8. Gokina NI, Bevan JA. Histamine-induced depolarization: ionic mechanisms and role in sustained contraction of rabbit cerebral arteries. Am J Physiol Heart Circ Physiol. 2000. 278:H2094–H2104.9. Gokina NI, Bevan JA. Role of intracellular Ca2+ release in histamine-induced depolarization in rabbit middle cerebral artery. Am J Physiol Heart Circ Physiol. 2000. 278:H2105–H2114.10. Austin C, Wray S. The effects of extracellular pH and calcium change on force and intracellular calcium in rat vascular smooth muscle. J Physiol. 1995. 488:281–291.
Article11. Rinaldi CJ, Amando Cattaneo E, Cigolani HE. Interaction between calcium and hydrogen ions in canine coronary arteries. J Mol Cell Cardiol. 1987. 19:773–784.12. Peng H-L, Jensen PE, Nilsson H, Aalkjaer C. Effect of acidosis on tension and [Ca2+]i in rat cerebral arteries: is there a role for membrane potential? Am J Physiol. 1998. 274:H655–H662.13. Oike M, Inoue Y, Kitamura K, Kuriyama H. Dual action of FRC8653, a novel dihydropyridine derivative, on the Ba2+ current recorded from the rabbit basilar artery. Circ Res. 1990. 67:993–1006.14. Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991. 12:349–354.15. Worley JF, Quayle JM, Standen NB, Nelson MT. Regulation of single calcium channels in cerebral arteries by voltage, serotonin, and dihydropyridines. Am J Physiol. 1991. 261:H1951–H1960.
Article16. West GA, Leppla DC, Simard JM. Effects of external pH on ionic currents in smooth muscle cells from the basilar artery of the guinea pig. Circ Res. 1992. 71:201–209.
Article17. Horie S, Yano S, Watanabe K. Intracellular alkalinization by NH4Cl increases cytosolic Ca2+ level and tension in the rat aortic smooth muscle. Life Sci. 1995. 56:1835–1843.18. Aoyama Y, Ueda K, Setogawa A, Kawai Y. Effects of pH on contraction and Ca2+ mobilization in vascular smooth muscles of the rabbit basilar artery. Jpn J Physiol. 1999. 49:55–62.19. Hamil OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.20. Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988. 92:145–159.
Article21. Isenberg G, Klöckner U. Calcium tolerant ventricular myocytes delivered by pre-incubation in a "KB-medium". Pflugers Arch. 1982. 395:6–18.22. Suh SH, Han JJ, Park SJ, Choi JY, Sim JH, Kim YC, Kim KW. Differentmechanisms for K+-induced relaxation in various artery. Korean J Physiol Pharmacol. 1998. 3:415–425.23. So I, Kang TM, Kim KW. Characteristics of Ca currents in rabbit basilar arterial smooth muscle cells. Seoul J Med. 1994. 35:169–182.24. Bolton TB. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979. 59:606–719.
Article25. Carl A, Lee HK, Sanders KM. Regulation of ion channels in smooth muscles by calcium. Am J Physiol. 1996. 271:C9–C34.
Article26. Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995. 47:387–573.27. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990. 259:C3–C18.
Article28. Garland CJ. The role of membrane depolarization in the contractile response of rabbit basilar arery to 5-hydroxytryptamine. J Physiol (Lond). 1987. 392:333–348.29. Karashima T, Kuriyama H. Electrical properties of smooth muscle cell membrane and neuromuscular transmission in the guinea-pig basilar artery. Br J Pharmacol. 1991. 74:495–504.
Article30. Sürprenant A, Neild TO, Holman ME. Membrane properties of rabbit basilar arteries and their responses to transmural stimulation. Pflugers Arch. 1987. 410:92–101.31. Ishikawa T, Hume JR, Keef KD. Modulation of K and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol. 1993. 468:379–400.32. Oike M, Kitamura K, Kuriyama H. Histamine H3-receptor activation augments voltage-dependent Ca2+ current via GTP hydrolysis in rabbitsaphenous artery. J Physiol. 1992. 448:133–152.33. Takagi T, Tan EC, Shibata S. Characteristics of histamine receptors in human cerebral arteries. Neurol Med Chir (Tokyo). 1993. 33:675–681.
Article34. Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Effect of acidosis on Ca2+-activated K+ channels in cultured porcine coronary artery smooth muscle cells. Pflugers Arch. 1998. 436:509–514.35. Shimamura K, Sekiguchi F, Sunano S. Tension oscillation in arteries and its abnormality in hypertensive animals. Clin Exp Pharmacol Physiol. 1999. 26:275–284.
Article36. Tostes RC, Storm DS, Chi DH, Webb RC. Intracellular calcium stores and oscillatory contractions in arteries from genetically hypertensive rats. Hypertens Res. 1996. 19:103–111.
Article37. Kang TM, So I, Kim KW. Caffeine- and histamine-induced oscillatons of K (Ca) current in single smooth muscle cells of rabbit cerebral artery. Pflugers Arch. 1995. 431:91–100.38. Gurevicius J, Salem MR, Metwally AA, Silver JM, Crystal GJ. Contribution of nitric oxide to coronary vasodilation during hypercapnic acidosis. Am J Physiol. 1995. 268(1 Pt 2):H39–H47.
Article39. Cowan CL, Cohen RA. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and independent responses. Am J Physiol. 1991. 261:H830–H835.
Article40. Vanhoutte PM. The end of the quest? Nature. 1987. 327:459–460.
Article41. Vanhoutte PM. Other endothelium-derived vasoactive factors. Circulation. 1993. 87:Suppl V. V9–V17.42. Nagao T, Vanhoutte PM. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992. 445:355–367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- he Changes of the Basilar Artery Following Experimental Cerebral Subarachnoidal Hemorrhage
- Microsurgical Anatomy of the Basilar Artery: Surgical Approaches to the Basilar Trunk and Vertebrobasilar Junction Aneurysms
- A Case of Moyamoya Disease Associated with Complete Duplication of the Basilar artery
- Changes in Myogenic Tone in Spontaneously Hypertensive Rat: Role of RhoA and Protein Kinase C
- The Effect of Low-dose Papaverine on In-vivo Rabbit Basilar Artery