Korean J Radiol.
2010 Apr;11(2):178-186. 10.3348/kjr.2010.11.2.178.
The Adjacent Vessel Sign on Breast MRI: New Data and a Subgroup Analysis for 1,084 Histologically Verified Cases
- Affiliations
-
- 1Institute of Diagnostic and Interventional Radiology, Friedrich-Schiller-University Jena, Erlanger Allee 101, D-07740 Jena, Germany. matthias.dietzel@med.uni-jena.de
- 2Institute of Pathology, Friedrich-Schiller-University Jena, Ziegelmuhlenweg 1, D-07740 Jena, Germany.
- 3Clinic of Gynecology, Friedrich-Schiller-University Jena, Bachstr. 18, D-07740 Jena, Germany.
- KMID: 1783194
- DOI: http://doi.org/10.3348/kjr.2010.11.2.178
Abstract
OBJECTIVE
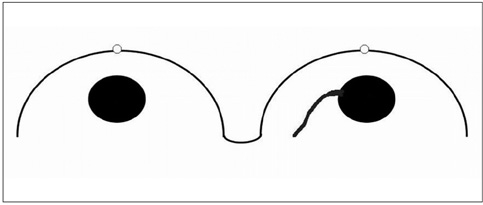
The adjacent vessel sign (AVS) is a descriptor for differentiating malignant from benign breast lesions on breast MRI (bMRI). This investigation was designed to verify the previous reports on the diagnostic accuracy of AVS and to assess correlation between AVS, histopathological diagnosis, lesion size and lesion grade.
MATERIALS AND METHODS
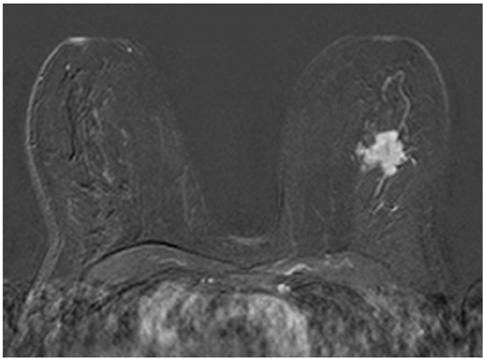
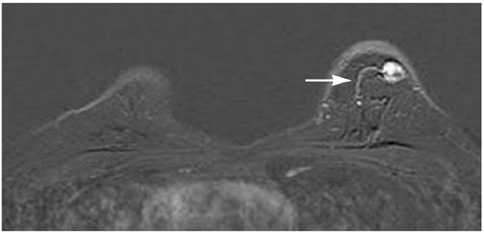
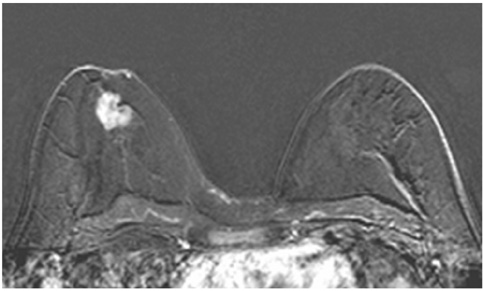
This study was approved by the local ethical committee. Experienced radiologists evaluated 1,084 lesions. The exclusion criteria were no histological verification after bMRI and breast interventions that were done up to one year before bMRI (surgery, core biopsy, chemo- or radiation therapy). The native and dynamic contrast-enhanced T1-weighted series were acquired using standardized protocols. The AVS was rated positive if a vessel leading to a lesion could be visualized. Prevalence of an AVS was correlated with the lesions' size, grade and histology using Chi-square-tests.
RESULTS
The AVS was significantly associated with malignancy (p < 0.001; sensitivity: 47%, specificity: 88%, positive-predictive-value [PPV]: 85%). Malignant lesions > 2 cm more often presented with an AVS than did those malignant lesions < 2 cm (p < 0.0001; sensitivity: 65%, PPV: 90%). There was no correlation of the AVS with the tumor grade. The prevalence of an AVS didn't significantly differ between invasive lobular carcinomas versus ductal carcinomas. In situ cancers were less frequently associated with an AVS (p < 0.001).
CONCLUSION
The adjacent vessel sign was significantly associated with malignancy. Thus, it can be used to accurately assess breast lesions on bMRI. In this study, the AVS was particularly associated with advanced and invasive carcinomas.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Breast/*pathology
Breast Neoplasms/*pathology
Contrast Media/diagnostic use
Diagnosis, Differential
Female
Gadolinium DTPA/diagnostic use
Humans
Image Enhancement/methods
Magnetic Resonance Imaging/*methods
Middle Aged
Neoplasms, Ductal, Lobular, and Medullary/*pathology
Observer Variation
Predictive Value of Tests
Reproducibility of Results
Sensitivity and Specificity
Young Adult
Figure
Cited by 1 articles
-
Prediction of Axillary Lymph Node Metastasis in Early Breast Cancer Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging and Diffusion-Weighted Imaging
Eun Ha Jeong, Eun Jung Choi, Hyemi Choi, Eun Hae Park, Ji Soo Song
Investig Magn Reson Imaging. 2019;23(2):125-135. doi: 10.13104/imri.2019.23.2.125.
Reference
-
1. Fischer DR, Malich A, Wurdinger S, Boettcher J, Dietzel M, Kaiser WA. The adjacent vessel on dynamic contrast-enhanced breast MRI. AJR Am J Roentgenol. 2006. 187:W147–W151.2. Fischer DR, Wurdinger S, Boettcher J, Malich A, Kaiser WA. Further signs in the evaluation of magnetic resonance mammography: a retrospective study. Invest Radiol. 2005. 40:430–435.3. Malich A, Fischer DR, Wurdinger S, Boettcher J, Marx C, Facius M, et al. Potential MRI interpretation model: differentiation of benign from malignant breast masses. AJR Am J Roentgenol. 2005. 185:964–970.4. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008. 246:116–124.5. Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008. 9:10–18.6. Ko EY, Han BK, Shin JH, Kang SS. Breast MRI for evaluating patients with metastatic axillary lymph node and initially negative mammography and sonography. Korean J Radiol. 2007. 8:382–389.7. Kim DY, Moon WK, Cho N, Ko ES, Yang SK, Park JS, et al. MRI of the breast for the detection and assessment of the size of ductal carcinoma in situ. Korean J Radiol. 2007. 8:32–39.8. Renz DM, Baltzer PA, Kullnig PE, Bottcher J, Vag T, Gajda M, et al. Clinical value of computer-assisted analysis in MR mammography. A comparison between two systems and three observers with different levels of experience. Rofo. 2008. 180:968–976.9. Williams TC, DeMartini WB, Partridge SC, Peacock S, Lehman CD. Breast MR imaging: computer-aided evaluation program for discriminating benign from malignant lesions. Radiology. 2007. 244:94–103.10. Thomas MA, Lipnick S, Velan SS, Liu X, Banakar S, Binesh N, et al. Investigation of breast cancer using two-dimensional MRS. NMR Biomed. 2009. 22:77–91.11. Mountford C, Ramadan S, Stanwell P, Malycha P. Proton MRS of the breast in the clinical setting. NMR Biomed. 2009. 22:54–64.12. Sardanelli F, Fausto A, Esseridou A, Di Leo G, Kirchin MA. Gadobenate dimeglumine as a contrast agent for dynamic breast magnetic resonance imaging: effect of higher initial enhancement thresholds on diagnostic performance. Invest Radiol. 2008. 43:236–242.13. Yabuuchi H, Matsuo Y, Okafuji T, Kamitani T, Soeda H, Setoguchi T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging. 2008. 28:1157–1165.14. Park JM, Park JH. Human in-vivo 31P MR spectroscopy of benign and malignant breast tumors. Korean J Radiol. 2001. 2:80–86.15. Vassiou K, Kanavou T, Vlychou M, Poultsidi A, Athanasiou E, Arvanitis DL, et al. Characterization of breast lesions with CEMR multimodal morphological and kinetic analysis: comparison with conventional mammography and high-resolution ultrasound. Eur J Radiol. 2009. 70:69–76.16. Tardivon AA, Athanasiou A, Thibault F, El Khoury C. Breast imaging and reporting data system (BIRADS): magnetic resonance imaging. Eur J Radiol. 2007. 61:212–215.17. Dietzel M, Baltzer PAT, Vag T, Gajda M, Camara O, Kaiser WA. The hook sign for differential diagnosis of malignant from benign lesions in magnetic resonance mammography - Experience in a study of 1084 histologically verified cases. Acta Radiol. 2010. 51:137–143.18. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.19. Armitage P, Berry G. Statistical methods in medical research. 1994. 3rd ed. London: Blackwell Scientific;131.20. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998. 17:857–872.21. Bland M. An introduction to medical statistics. 2000. Oxford: Oxford University Press.22. Langer SA, Horst KC, Ikeda DM, Daniel BL, Kong CS, Dirbas FM. Pathologic correlates of false positive breast magnetic resonance imaging findings: which lesions warrant biopsy? Am J Surg. 2005. 190:633–640.23. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.24. Bässler R. Remmele W, editor. Mamma. Pathologie. 1997. Berlin: Springer;135–365.25. Fridman V, Humblet C, Bonjean K, Boniver J. Assessment of tumor angiogenesis in invasive breast carcinomas: absence of correlation with prognosis and pathological factors. Virchows Arch. 2000. 437:611–617.26. Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992. 84:1875–1887.27. Westerhof JP, Fischer U, Moritz JD, Oestmann JW. MR imaging of mammographically detected clustered microcalcifications: is there any value? Radiology. 1998. 207:675–681.28. Gilles R, Meunier M, Lucidarme O, Zafrani B, Guinebretiere JM, Tardivon AA, et al. Clustered breast microcalcifications: evaluation by dynamic contrast-enhanced subtraction MRI. J Comput Assist Tomogr. 1996. 20:9–14.29. Brinck U, Fischer U, Korabiowska M, Jutrowski M, Schauer A, Grabbe E. The variability of fibroadenoma in contrast-enhanced dynamic MR mammography. AJR Am J Roentgenol. 1997. 168:1331–1334.30. Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992. 267:10931–10934.31. Kaiser WA. MR mammography--a critical stocktaking. Rofo. 1996. 165:425–427.32. Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999. 213:881–888.33. Nunes LW, Schnall MD, Orel SG, Hochman MG, Langlotz CP, Reynolds CA, et al. Breast MR imaging: interpretation model. Radiology. 1997. 202:833–841.34. Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007. 244:672–691.35. Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter? Positive predictive value of MRI-detected breast lesions as a function of lesion size. AJR Am J Roentgenol. 2006. 186:426–443.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic value of magnetic resonance imaging using superparamagnetic iron oxide for axillary node metastasis in patients with breast cancer: a meta-analysis
- Usefulness of Breast MRI for Diagnosing an Extensive Intraductal Component of Breast Cancer: Comparison with Mammography and Ultrasonography
- Characteristic Signs on T2*-Based Imaging and Their Relationship with Results of Reperfusion Therapy for Acute Ischemic Stroke: A Systematic Review and Evidence to Date
- Clinical Applications of Breast MRI
- MR Findings of Hamartoma of the Breast: A Report of Two Cases