Yonsei Med J.
2012 Jan;53(1):228-230. 10.3349/ymj.2012.53.1.228.
Toxic Epidermal Necrolysis with Ocular Involvement Following Vaccination for Hemorrhagic Fever with Renal Syndrome
- Affiliations
-
- 1Department of Ophthalmology, Armed Forces Capital Hospital, Seongnam, Korea. lsm10003@chol.com
- 2Department of Dermatology, Armed Forces Capital Hospital, Seongnam, Korea.
- 3Department of Ophthalmology, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- KMID: 1779712
- DOI: http://doi.org/10.3349/ymj.2012.53.1.228
Abstract
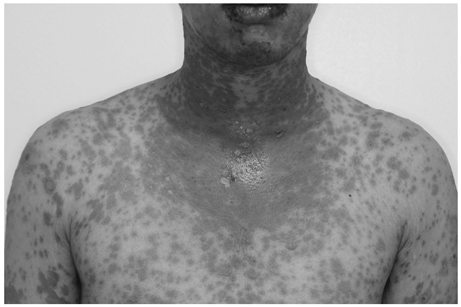
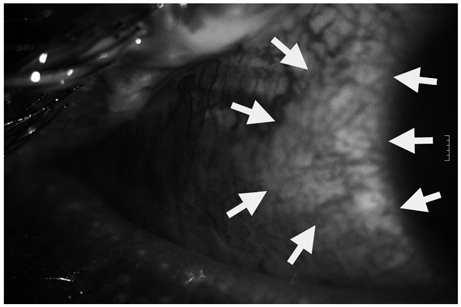
- We report a case of toxic epidermal necrolysis with ocular involvement following vaccination for hemorrhagic fever with renal syndrome. A healthy 20-year-old male soldier presented with confluent purpuric and erythematous dusky red macules evolving to flaccid blister and epidermal detachment on the whole body with conjunctival injection. The patient had no antecedent medical or surgical conditions except for two doses of hemorrhagic fever with renal syndrome vaccination. With supportive care, skin lesions were improved. Ophthalmic examinations revealed conjunctival injection with epithelial defects in both eyes. Ocular complications were resolved after amniotic membrane transplantation. Toxic epidermal necrolysis may be considered as a possible complication of hemorrhagic fever with renal syndrome vaccination.
MeSH Terms
Figure
Reference
-
1. Power WJ, Ghoraishi M, Merayo-Lloves J, Neves RA, Foster CS. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. 1995. 102:1669–1676.
Article2. Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol. 1987. 123:1160–1165.
Article3. Chang YS, Huang FC, Tseng SH, Hsu CK, Ho CL, Sheu HM. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea. 2007. 26:123–129.
Article4. López-García JS, Rivas Jara L, García-Lozano CI, Conesa E, de Juan IE, Murube del Castillo J. Ocular features and histopathologic changes during follow-up of toxic epidermal necrolysis. Ophthalmology. 2011. 118:265–271.
Article5. Sotozono C, Ueta M, Koizumi N, Inatomi T, Shirakata Y, Ikezawa Z, et al. Diagnosis and treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis with ocular complications. Ophthalmology. 2009. 116:685–690.
Article6. Gueudry J, Roujeau JC, Binaghi M, Soubrane G, Muraine M. Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Arch Dermatol. 2009. 145:157–162.
Article7. Katoulis AC, Liakou A, Bozi E, Theodorakis M, Alevizou A, Zafeiraki A, et al. Erythema multiforme following vaccination for human papillomavirus. Dermatology. 2010. 220:60–62.
Article8. Chopra A, Drage LA, Hanson EM, Touchet NL. Stevens-Johnson syndrome after immunization with smallpox, anthrax, and tetanus vaccines. Mayo Clin Proc. 2004. 79:1193–1196.
Article9. Kaur S, Handa S. Erythema multiforme following vaccination in an infant. Indian J Dermatol Venereol Leprol. 2008. 74:251–253.
Article10. Ball R, Ball LK, Wise RP, Braun MM, Beeler JA, Salive ME. Stevens-Johnson syndrome and toxic epidermal necrolysis after vaccination: reports to the vaccine adverse event reporting system. Pediatr Infect Dis J. 2001. 20:219–223.
Article11. Schmaljohn C. Vaccines for hantaviruses. Vaccine. 2009. 27:Suppl 4. D61–D64.
Article12. Sohn YM, Rho HO, Park MS, Kim JS, Summers PL. Primary humoral immune responses to formalin inactivated hemorrhagic fever with renal syndrome vaccine (Hantavax): consideration of active immunization in South Korea. Yonsei Med J. 2001. 42:278–284.
Article13. Bi Z, Formenty PB, Roth CE. Hantavirus infection: a review and global update. J Infect Dev Ctries. 2008. 2:3–23.
Article14. Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. 2009. 54:686–696.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Complete Vaginal Ostruction Following Toxic Epidermal Necrolysis
- A Case of Carbamazepine- Induced- Toxic Epidermal Necrolysis
- A Case of Toxic Epidermal Necrolysis Due to Contact of Paraquat(Gramoxone(R))
- T Cell Mediated Drug Hypersensitivity: Stevens Johnson Syndrome and Toxic Epidermal Necrolysis
- Toxic Epidermal Necrolysis Induced by the Topical Carbonic Anhydrase Inhibitors Brinzolamide and Dorzolamide