Yonsei Med J.
2012 Jan;53(1):43-52. 10.3349/ymj.2012.53.1.43.
Statistical Mapping Analysis of Brain Metabolism in Patients with Subcortical Aphasia after Intracerebral Hemorrhage: A Pilot Study of F-18 FDG PET Images
- Affiliations
-
- 1Department and Research Institute of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Korea. ywkim1@yuhs.ac
- 2Department of Physical Medicine and Rehabilitation, National Health Insurance Corporation Ilsan Hospital, Ilsan, Korea.
- 3Department of Nuclear Medicine, Ajou University School of Medicine, Suwon, Korea.
- KMID: 1779685
- DOI: http://doi.org/10.3349/ymj.2012.53.1.43
Abstract
- PURPOSE
This study was aimed to evaluate the brain metabolism in patients with subcortical aphasia after intracerebral hemorrhage (ICH) and the relationship between the severity of aphasia and regional brain metabolism, by using statistical mapping analysis of F-18 fluorodeoxyglucose positron emission tomography (F-18 FDG PET) images.
MATERIALS AND METHODS
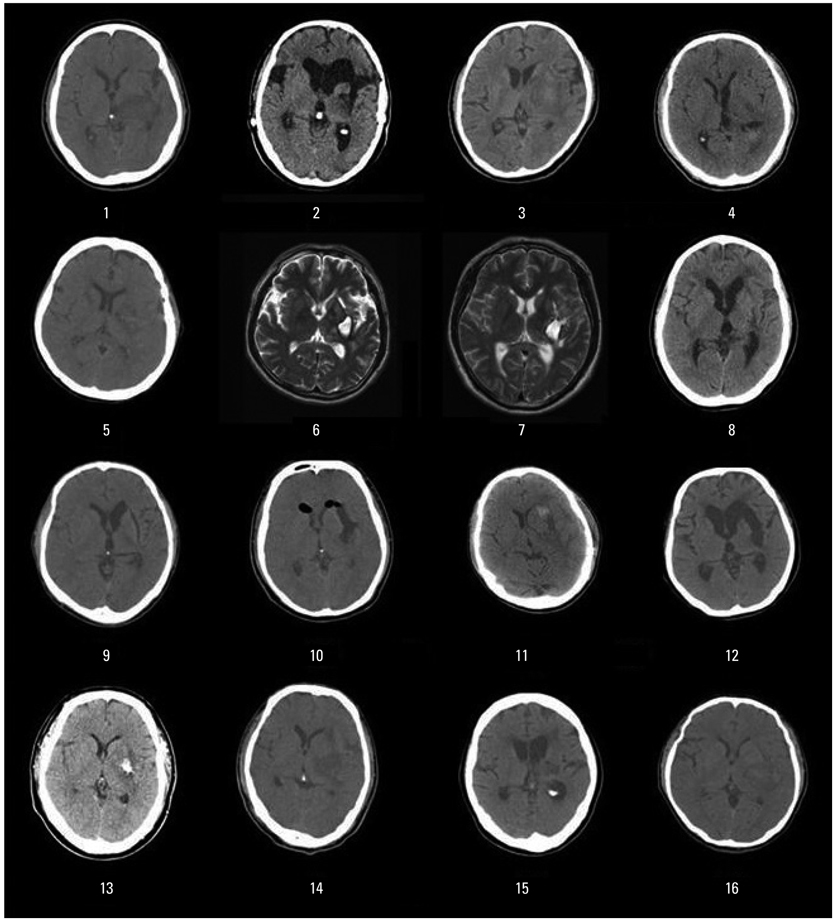
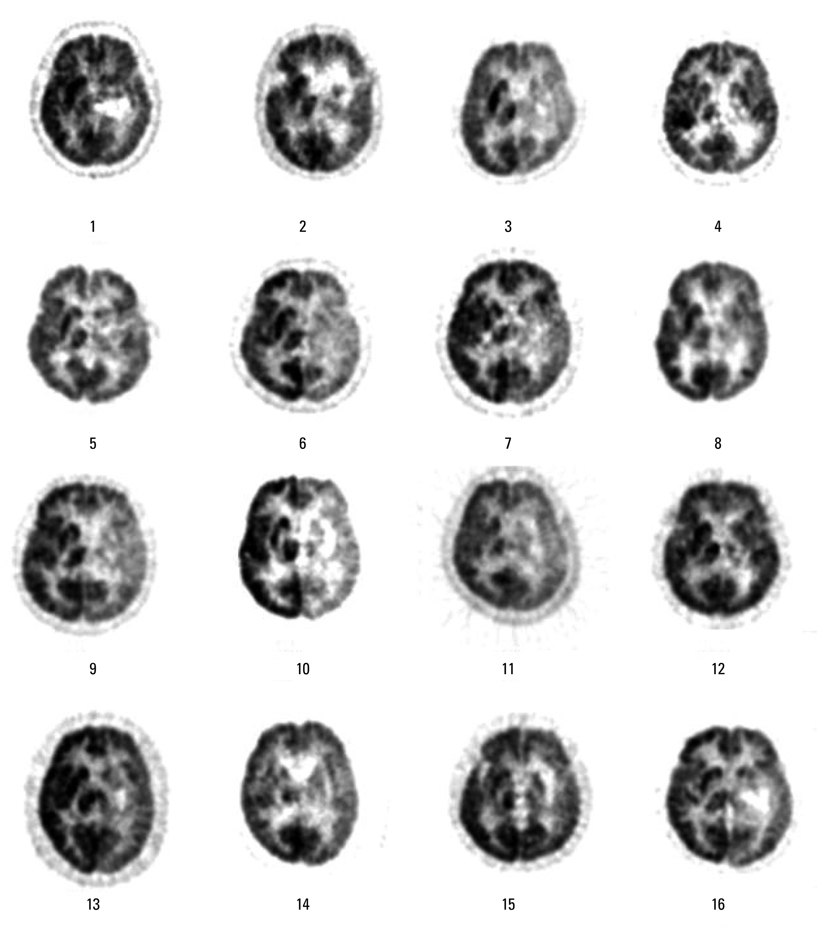
Sixteen right-handed Korean speaking patients with subcortical aphasia following ICH were enrolled. All patients underwent Korean version of the Western Aphasia Battery and the brain F-18 FDG PET study. Using statistical parametric mapping analysis, we compared the brain metabolisms shown on F-18 FDG PET from 16 patients with subcortical aphasia and 16 normal controls. In addition, we investigated the relationship between regional brain metabolism and the severity of aphasia using covariance model.
RESULTS
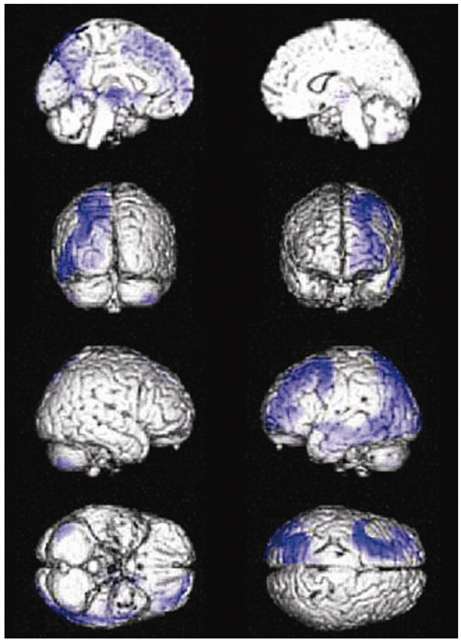
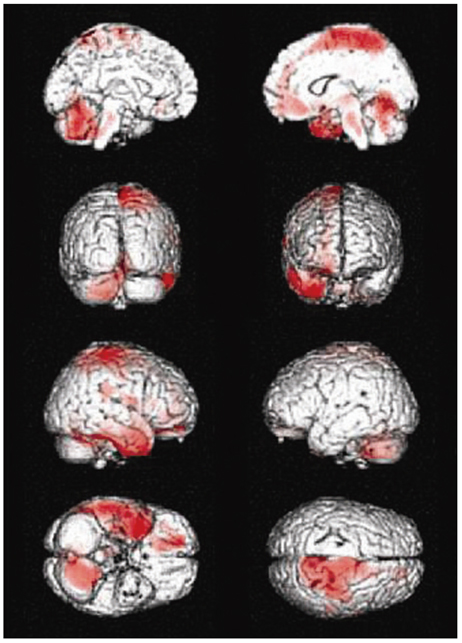
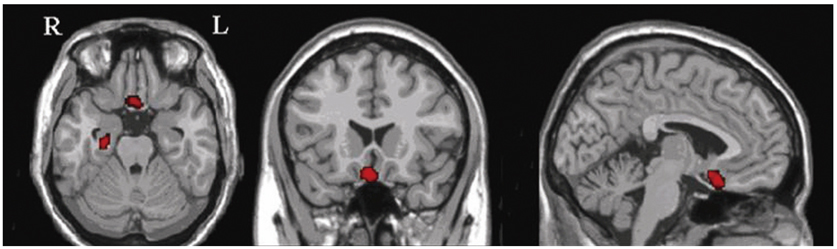
Compared to the normal controls, subcortical aphasia after ICH showed diffuse hypometabolism in the ipsilateral cerebrum (frontal, parietal, temporal, occipital, putamen, thalamus) and in the contralateral cerebellum (P corrected <0.001), and showed diffuse hypermetabolism in the contralateral cerebrum (frontal, parietal, temporal) and in the ipsilateral cerebellum (P FDR corrected <0.001). In the covariance analysis, increase of aphasia quotient was significantly correlated with increased brain metabolism in the both orbitofrontal cortices, the right hippocampal and the right parahippocampal cortices (P uncorrected <0.01).
CONCLUSION
Our findings suggest that frontal, parietal, and temporal cortices, which are parts of neural network for cognition, may have a supportive role for language performance in patients with subcortical aphasia after ICH.
Keyword
MeSH Terms
-
Adult
Aged
Aphasia/etiology/metabolism/*radionuclide imaging
Brain/metabolism/*radionuclide imaging
Brain Mapping/*methods
Cerebral Hemorrhage/complications/metabolism/*radionuclide imaging
Female
Fluorodeoxyglucose F18/*diagnostic use
Humans
Male
Middle Aged
Pilot Projects
Positron-Emission Tomography/*methods
Figure
Reference
-
1. Damasio AR, Damasio H, Rizzo M, Varney N, Gersh F. Aphasia with nonhemorrhagic lesions in the basal ganglia and internal capsule. Arch Neurol. 1982. 39:15–24.
Article2. de Boissezon X, Démonet JF, Puel M, Marie N, Raboyeau G, Albucher JF, et al. Subcortical aphasia: a longitudinal PET study. Stroke. 2005. 36:1467–1473.3. Perani D, Vallar G, Cappa S, Messa C, Fazio F. Aphasia and neglect after subcortical stroke. A clinical/cerebral perfusion correlation study. Brain. 1987. 110:1211–1229.4. Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002. 125:1094–1104.
Article5. Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997. 58:355–402.
Article6. Cappa SF, Perani D, Grassi F, Bressi S, Alberoni M, Franceschi M, et al. A PET follow-up study of recovery after stroke in acute aphasics. Brain Lang. 1997. 56:55–67.
Article7. Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999. 66:155–161.
Article8. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002. 15:273–289.
Article9. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971. 9:97–113.
Article10. Kim H, Na DL. Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol. 2004. 26:1011–1020.
Article11. Kertesz A. Aphasia and associated disorders: taxonomy, localization and recovery. 1979. Orlando, FL: Grune & Stratton.12. Kertesz A, Poole E. The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci. 1974. 1:7–16.
Article13. Kim YW, Shin JC, An YS. Changes in cerebral glucose metabolism in patients with posttraumatic cognitive impairment after memantine therapy: a preliminary study. Ann Nucl Med. 2010. 24:363–369.
Article14. Kim YW, Shin JC, An YS. Effects of methylphenidate on cerebral glucose metabolism in patients with impaired consciousness after acquired brain injury. Clin Neuropharmacol. 2009. 32:335–339.
Article15. Kreisler A, Godefroy O, Delmaire C, Debachy B, Leclercq M, Pruvo JP, et al. The anatomy of aphasia revisited. Neurology. 2000. 54:1117–1123.
Article16. Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000. 55:1883–1894.
Article17. Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bézy C, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008. 70:290–298.
Article18. Heiss WD, Thiel A, Kessler J, Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage. 2003. 20:Suppl 1. S42–S49.
Article19. Crinion JT, Leff AP. Recovery and treatment of aphasia after stroke: functional imaging studies. Curr Opin Neurol. 2007. 20:667–673.
Article20. Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005. 93:95–105.
Article21. Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006. 98:118–123.
Article22. Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008. 105:18035–18040.
Article23. Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Hum Brain Mapp. 2008. 29:581–593.
Article24. Horner J, Dawson DV, Heyman A, Fish AM. The usefulness of the Western Aphasia Battery for differential diagnosis of Alzheimer dementia and focal stroke syndromes: preliminary evidence. Brain Lang. 1992. 42:77–88.
Article25. Kim H, Na DL. Korean version of the Western Aphasia Battery. 2001. Seoul, Korea: Paradise Welfare Foundation, Institute for Children with Disabilities.26. Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992. 323:341–358.
Article27. Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009. 87:149–174.28. Rowland DC, Kentros CG. Potential anatomical basis for attentional modulation of hippocampal neurons. Ann N Y Acad Sci. 2008. 1129:213–224.
Article29. Crosson B. Subcortical functions in language: a working model. Brain Lang. 1985. 25:257–292.
Article30. D'Esposito M, Alexander MP. Subcortical aphasia: distinct profiles following left putaminal hemorrhage. Neurology. 1995. 45:38–41.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Subcortical Aphasia in Stroke Patients
- Post-operative Changes of Cerebral Glucose Metabolism in Patients with Lumbar Spinal Stenosis with Pre-operative Anxiety: Statistical Parametric Mapping Analysis of F-18 FDG Brain PET
- Clinical study of subcortical aphasia using brain SPECT and neurolinguistical methods
- F-18 FDG PET Images of the Cervix at Various Time Points after the Loop Electrosurgical Excision Procedure
- Effect of Harderian adenectomy on the statistical analyses of mouse brain imaging using positron emission tomography