Yonsei Med J.
2010 Jan;51(1):39-44. 10.3349/ymj.2010.51.1.39.
Helicobacter pylori Urease Activity is Influenced by Ferric Uptake Regulator
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. i101016@skku.edu
- KMID: 1779604
- DOI: http://doi.org/10.3349/ymj.2010.51.1.39
Abstract
- PURPOSE
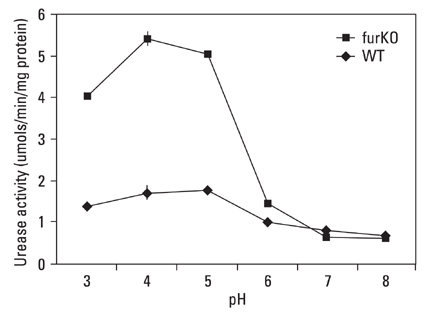
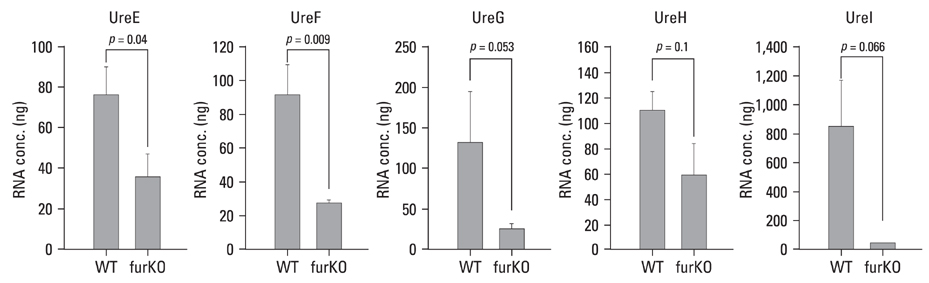
The role of the Ferric Uptake Regulator (FUR) in the acid resistance of Helicobacter pylori (H. pylori) has been thought to be independent of urease. However, we demonstrated in this study that Fur influences urease activity. MATERIALS AND METHODS: A fur knockout mutant of H. pylori was constructed by replacing the Fur gene with a kanamycin resistant marker gene. The wild-type H. pylori and fur mutant were compared for survival. The integrity of the inner membrane of the bacteria was evaluated by confocal microscopy using membrane-permeant and -impermeant fluorescent DNA probes. Urease activity of intact H. pylori was measured between pH 3 and 8. Real time PCR of both strains was performed for urease genes including ureI, ureE, ureF, ureG, and ureH. RESULTS: The fur deletion affected the survival of H. pylori at pH 4. The urease activity curve of the intact fur mutant showed the same shape as the wild-type but was 3-fold lower than the wild-type at a pH of less than 5. Real time PCR revealed that the expression of all genes was consistently down-regulated in the fur mutant. CONCLUSION: The results of this study showed that fur appears to be involved in acid resistant H. pylori urease activity.
Keyword
MeSH Terms
Figure
Reference
-
1. Chu S, Tanaka S, Kaunitz JD, Montrose MH. Dynamic regulation of gastric surface pH by luminal pH. J Clin Invest. 1999. 103:605–612.
Article2. Quigley EM, Turnberg LA. pH of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology. 1987. 92:1876–1884.
Article3. Schade C, Flemström G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994. 107:180–188.
Article4. Bauerfeind P, Garner R, Dunn BE, Mobley HL. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut. 1997. 40:25–30.
Article5. van Vliet AH, Kuipers EJ, Stoof J, Poppelaars SW, Kusters JG. Acid-responsive gene induction of ammonia-producing enzymes in Helicobacter pylori is mediated via a metal-responsive repressor cascade. Infect Immun. 2004. 72:766–773.6. Hawtin PR, Stacey AR, Newell DG. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990. 136:1995–2000.7. Phadnis SH, Dunn BE. Surface localization of H. pylori urease and Hp54K requires bacterial lysis (abstr). Gut. 1995. 37:A19.8. Scott DR, Weeks D, Hong C, Postius S, Melchers K, Sachs G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology. 1998. 114:58–70.
Article9. Modlin IM, Sachs G. Acid Related Diseases; Biology and Treatment. 2004. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;459–478.10. Scott DR, Marcus EA, Weeks DL, Sachs G. Mechanisms of acid resistance due to the urease system of Helicobacter pylori. Gastroenterology. 2002. 123:187–195.
Article11. Rektorschek M, Buhmann A, Weeks D, Schwan D, Bensch KW, Eskandari S, et al. Acid resistance of Helicobacter pylori depends on the UreI membrane protein and an inner membrane proton barrier. Mol Microbiol. 2000. 36:141–152.
Article12. Bijlsma JJ, Gerrits MM, Imamdi R, Vandenbroucke-Grauls CM, Kusters JG. Urease-positive, acid-sensitive mutants of Helicobacter pylori: urease-independent acid resistance involved in growth at low pH. FEMS Microbiol Lett. 1998. 167:309–313.
Article13. Bijlsma JJ, Lie-A-Ling M, Nootenboom IC, Vandenbroucke-Grauls CM, Kusters JG. Identification of loci essential for the growth of Helicobacter pylori under acidic conditions. J Infect Dis. 2000. 182:1566–1569.
Article14. Andrews SC, Robinson AK, Rodriguez-Quinoñes F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003. 27:215–237.
Article15. Hall HK, Foster JW. The role of fur in the acid tolerance response of Salmonella typhimurium is physiologically and genetically separable from its role in iron acquisition. J Bacteriol. 1996. 178:5683–5691.
Article16. Bijlsma JJ, Waidner B, Vliet AH, Hughes NJ, Häg S, Bereswill S, et al. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect Immun. 2002. 70:606–611.
Article17. van Vliet AH, Stoof J, Poppelaars SW, Bereswill S, Homuth G, Kist M, et al. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori fur repressor. J Biol Chem. 2003. 278:9052–9057.
Article18. Bury-Moné S, Skouloubris S, Dauga C, Thiberge JM, Dailidiene D, Berg DE, et al. Presence of active aliphatic amidases in Helicobacter species able to colonize the stomach. Infect Immun. 2003. 71:5613–5622.
Article19. Merrell DS, Goodrich ML, Otto G, Tompkins LS, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun. 2003. 71:3529–3539.
Article20. Skouloubris S, Labigne A, De Reuse H. The AmiE aliphatic amidase and AmiF formamidase of Helicobacter pylori: natural evolution of two enzyme paralogues. Mol Microbiol. 2001. 40:596–609.
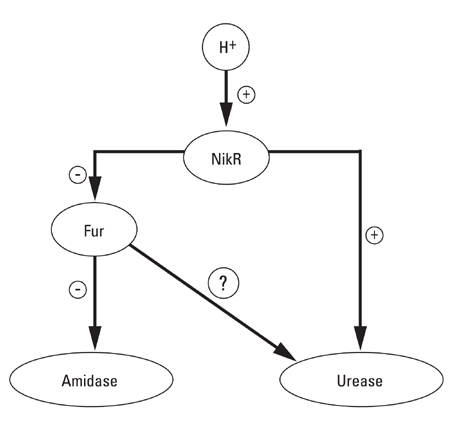
Article21. van Vliet AH, Ernst FD, Kusters JG. NikR-mediated regulation of Helicobacter pylori acid adaptation. Trends Microbiol. 2004. 12:489–494.
Article22. Rosen S, Skaletsky H. Krawetz SA, Misener S, editors. Primer3 on the WWW for general users and for biologist programmers. Bioinformatics Methods and Protocols: Methods in Molecular Biology. 2000. Totowa, NJ: Humana Press;365–386.23. Tsuda M, Karita M, Morshed MG, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994. 62:3586–3589.24. Moran AP, Knirel YA, Senchenkova SN, Widmalm G, Hynes SO, Jansson PE. Phenotypic variation in molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. Acid-induced phase variation in Lewis(x) and Lewis(y) expression by H. Pylori lipopolysaccharides. J Biol Chem. 2002. 277:5785–5795.25. Huesca M, Goodwin A, Bhagwansingh A, Hoffman P, Lingwood CA. Characterization of an acidic-pH-inducible stress protein (hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori. Infect Immun. 1998. 66:4061–4067.
Article26. van Vliet AH, Kuipers EJ, Waidner B, Davies BJ, de Vries N, Penn CW, et al. Nickel-responsive induction of urease expression in Helicobacter pylori is mediated at the transcriptional level. Infect Immun. 2001. 69:4891–4897.
Article27. Bury-Moné S, Thiberge JM, Contreras M, Maitournam A, Labigne A, De Reuse H. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol Microbiol. 2004. 53:623–638.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Purification of the urease of helicobacter pylori and production of monoclonal antibody to the urease of helicobacter pylori
- Development of diagnostic method of helicobacter pylori infection: I. molecular cloning and DNA sequencing of urease
- Serologic Diagnosis of Helicobacter pylori Gastritis in Children : Seroepidemiology of H. pylori in Normal School Children and Diagnostic Accuracy of IgG GAP Test in Children with Gastrointestinal Symptoms
- Histological Changes of Gastric Mucosa after Helicobacter pylori Eradication
- Activation of Urease Apoprotein of Helicobacter pylori