Yonsei Med J.
2010 Jan;51(1):9-17. 10.3349/ymj.2010.51.1.9.
Intrinsic Cellular Defenses against Virus Infection by Antiviral Type I Interferon
- Affiliations
-
- 1BioTherapeutics Engineering Laboratory, Department of Genetic Engineering, Sungkyunkwan University, Suwon, Korea. jsyang@skku.edu
- KMID: 1779600
- DOI: http://doi.org/10.3349/ymj.2010.51.1.9
Abstract
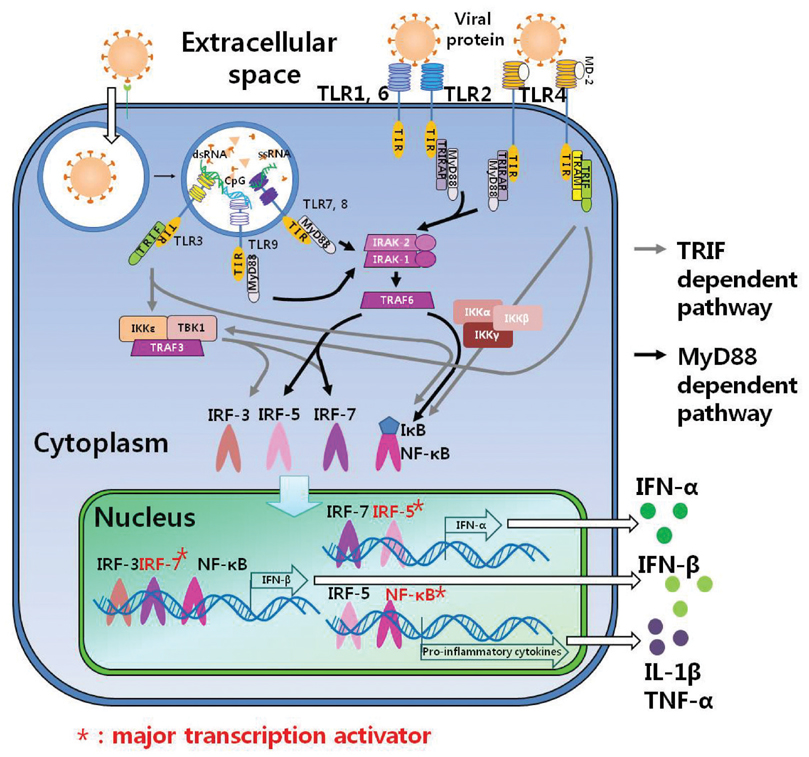
- Intrinsic cellular defenses are non-specific antiviral activities by recognizing pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs), one of the pathogen recognize receptor (PRR), sense various microbial ligands. Especially, TLR2, TLR3, TLR4, TLR7, TLR8 and TLR9 recognize viral ligands such as glycoprotein, single- or double-stranded RNA and CpG nucleotides. The binding of viral ligands to TLRs transmits its signal to Toll/interleukin-1 receptor (TIR) to activate transcription factors via signal transduction pathway. Through activation of transcription factors, such as interferon regulatory factor-3, 5, and 7 (IRF-3, 5, 7) or nuclear factor-kappaB (NF-kappaB), type I interferons are induced, and antiviral proteins such as myxovirus-resistance protein (Mx) GTPase, RNA-dependent Protein Kinase (PKR), ribonuclease L (RNase L), Oligo-adenylate Synthetase (OAS) and Interferon Stimulated Gene (ISG) are further expressed. These antiviral proteins play an important role of antiviral resistancy against several viral pathogens in infected cells and further activate innate immune responses.
MeSH Terms
Figure
Cited by 2 articles
-
COVID-19 Patients Upregulate Toll-like Receptor 4-mediated Inflammatory Signaling That Mimics Bacterial Sepsis
Kyung Mok Sohn, Sung-Gwon Lee, Hyeon Ji Kim, Shinhyea Cheon, Hyeongseok Jeong, Jooyeon Lee, In Soo Kim, Prashanta Silwal, Young Jae Kim, Seungwha Paik, Chaeuk Chung, Chungoo Park, Yeon-Sook Kim, Eun-Kyeong Jo
J Korean Med Sci. 2020;35(38):e343. doi: 10.3346/jkms.2020.35.e343.Adenovirus as a new agent for multiple myeloma therapies: Opportunities and restrictions
Svjetlana Raus, Silvia Coin, Vladia Monsurrò
Korean J Hematol. 2011;46(4):229-238. doi: 10.5045/kjh.2011.46.4.229.
Reference
-
1. Wang JP, Liu P, Latz E, Golenbock DT, Finberg RW, Libraty DH. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol. 2006. 177:7114–7121.2. Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008. 82:7613–7623.3. Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007. 179:3171–3177.
Article4. Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006. 103:17343–17348.
Article5. Bell JK, Askins J, Hall PR, Davies DR, Segal DM. The dsRNA binding site of human Toll-like receptor 3. Proc Natl Acad Sci U S A. 2006. 103:8792–8797.
Article6. Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991. 351:355–356.
Article7. Buchanan SG, Gay NJ. Structural and functional diversity in the leucine-rich repeat family of proteins. Prog Biophys Mol Biol. 1996. 65:1–44.
Article8. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998. 95:588–593.9. Kubarenko A, Frank M, Weber AN. Structure-function relationships of Toll-like receptor domains through homology modelling and molecular dynamics. Biochem Soc Trans. 2007. 35:1515–1518.
Article10. Yonkers NL, Rodriguez B, Milkovich KA, Asaad R, Lederman MM, Heeger PS, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007. 178:4436–4444.11. Sirén J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005. 174:1932–1937.
Article12. Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, et al. TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem. 2006. 281:21013–21021.13. Sarkar SN, Elco CP, Peters KL, Chattopadhyay S, Sen GC. Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. J Biol Chem. 2007. 282:3423–3427.
Article14. Andreakos E, Sacre SM, Smith C, Lundberg A, Kiriakidis S, Stonehouse T, et al. Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004. 103:2229–2237.
Article15. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001. 413:732–738.16. Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006. 439:204–207.17. Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006. 13:816–825.
Article18. Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000. 1:419–424.
Article19. Bracci L, Canini I, Venditti M, Spada M, Puzelli S, Donatelli I, et al. Type I IFN as a vaccine adjuvant for both systemic and mucosal vaccination against influenza virus. Vaccine. 2006. 24:Suppl 2. S2-56–S2-57.
Article20. Johnson GA, Joyce MM, Yankey SJ, Hansen TR, Ott TL. The Interferon Stimulated Genes (ISG) 17 and Mx have different temporal and spatial expression in the ovine uterus suggesting more complex regulation of the Mx gene. J Endocrinol. 2002. 174:R7–R11.
Article21. Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006. 176:1733–1740.
Article22. Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006. 2:e53.
Article23. Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly (I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003. 278:16713–16719.
Article24. Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005. 280:5571–5580.25. Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002. 76:8729–8736.
Article26. Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005. 175:4189–4193.27. Reske A, Pollara G, Krummenacher C, Katz DR, Chain BM. Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J Immunol. 2008. 180:7525–7536.
Article28. Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003. 77:4588–4596.
Article29. Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004. 127:1513–1524.30. da Silva Correia J, Ulevitch RJ. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem. 2002. 277:1845–1854.
Article31. Monick MM, Yarovinsky TO, Powers LS, Butler NS, Carter AB, Gudmundsson G, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003. 278:53035–53044.
Article32. Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006. 80:866–874.
Article33. Thomas A, Laxton C, Rodman J, Myangar N, Horscroft N, Parkinson T. Investigating Toll-like receptor agonists for potential to treat hepatitis C virus infection. Antimicrob Agents Chemother. 2007. 51:2969–2978.
Article34. Jude BA, Pobezinskaya Y, Bishop J, Parke S, Medzhitov RM, Chervonsky AV, et al. Subversion of the innate immune system by a retrovirus. Nat Immunol. 2003. 4:573–578.
Article35. Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002. 99:2281–2286.
Article36. Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005. 174:4043–4050.
Article37. Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006. 36:3256–3267.
Article38. Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004. 303:1526–1529.
Article39. Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005. 26:221–229.
Article40. Kandimalla ER, Zhu FG, Bhagat L, Yu D, Agrawal S. Toll-like receptor 9: modulation of recognition and cytokine induction by novel synthetic CpG DNAs. Biochem Soc Trans. 2003. 31:654–658.
Article41. Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, et al. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem. 2005. 280:17005–17012.
Article42. La Ferla K, Reimann C, Jelkmann W, Hellwig-Bürgel T. Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-kappaB. FASEB J. 2002. 16:1811–1813.43. Romieu-Mourez R, Solis M, Nardin A, Goubau D, Baron-Bodo V, Lin R, et al. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res. 2006. 66:10576–10585.
Article44. Barnes BJ, Kellum MJ, Field AE, Pitha PM. Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes. Mol Cell Biol. 2002. 22:5721–5740.
Article45. Barnes BJ, Field AE, Pitha-Rowe PM. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J Biol Chem. 2003. 278:16630–16641.
Article46. Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005. 201:1435–1446.
Article47. Cutrone EC, Langer JA. Identification of critical residues in bovine IFNAR-1 responsible for interferon binding. J Biol Chem. 2001. 276:17140–17148.
Article48. Dumler I, Weis A, Mayboroda OA, Maasch C, Jerke U, Haller H, et al. The Jak/Stat pathway and urokinase receptor signaling in human aortic vascular smooth muscle cells. J Biol Chem. 1998. 273:315–321.
Article49. Chang YZ, Lei YC, Hao YH, Chen SS, Wu W, Yang DL, et al. [Study of the effect of hepatitis C virus core protein on interferoninduced antiviral genes expression and its mechanisms]. Sheng Wu Gong Cheng Xue Ba. 2007. 23:1000–1004.50. Haller O, Arnheiter H, Lindenmann J, Gresser I. Host gene influences sensitivity to interferon action selectively for influenza virus. Nature. 1980. 283:660–662.
Article51. Koerner I, Kochs G, Kalinke U, Weiss S, Staeheli P. Protective role of beta interferon in host defense against influenza A virus. J Virol. 2007. 81:2025–2030.
Article52. Lee SH, Vidal SM. Functional diversity of Mx proteins: variations on a theme of host resistance to infection. Genome Res. 2002. 12:527–530.
Article53. Kochs G, Haller O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc Natl Acad Sci U S A. 1999. 96:2082–2086.
Article54. Davis S, Watson JC. In vitro activation of the interferon-induced, double-stranded RNA-dependent protein kinase PKR by RNA from the 3' untranslated regions of human alpha-tropomyosin. Proc Natl Acad Sci U S A. 1996. 93:508–513.
Article55. Cheng G, Yang K, He B. Dephosphorylation of eIF-2alpha mediated by the gamma(1)34.5 protein of herpes simplex virus type 1 is required for viral response to interferon but is not sufficient for efficient viral replication. J Virol. 2003. 77:10154–10161.
Article56. Montero H, Rojas M, Arias CF, López S. Rotavirus infection induces the phosphorylation of eIF2alpha but prevents the formation of stress granules. J Virol. 2008. 82:1496–1504.
Article57. García MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007. 89:799–811.
Article58. García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006. 70:1032–1060.59. Carr DJ, Tomanek L, Silverman RH, Campbell IL, Williams BR. RNA-dependent protein kinase is required for alpha-1 interferon transgene-induced resistance to genital herpes simplex virus type 2. J Virol. 2005. 79:9341–9345.
Article60. Austin BA, James C, Silverman RH, Carr DJ. Critical role for the oligoadenylate synthetase/RNase L pathway in response to IFN-beta during acute ocular herpes simplex virus type 1 infection. J Immunol. 2005. 175:1100–1106.
Article61. Kumar R, Choubey D, Lengyel P, Sen GC. Studies on the role of the 2'-5'-oligoadenylate synthetase-RNase L pathway in beta interferon-mediated inhibition of encephalomyocarditis virus replication. J Virol. 1988. 62:3175–3181.
Article62. Kodym R, Kodym E, Story MD. 2'-5'-Oligoadenylate synthetase is activated by a specific RNA sequence motif. Biochem Biophys Res Commun. 2009. 388:317–322.
Article63. Kerr IM, Brown RE. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978. 75:256–260.
Article64. Malathi K, Dong B, Gale M Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007. 448:816–819.
Article65. Dong B, Xu L, Zhou A, Hassel BA, Lee X, Torrence PF, et al. Intrinsic molecular activities of the interferon-induced 2-5A-dependent RNase. J Biol Chem. 1994. 269:14153–14158.
Article66. Hsiang TY, Zhao C, Krug RM. Interferon-induced ISG15 conjugation inhibits influenza A virus gene expression and replication in human cells. J Virol. 2009. 83:5971–5977.
Article67. Espert L, Degols G, Lin YL, Vincent T, Benkirane M, Mechti N. Interferon-induced exonuclease ISG20 exhibits an antiviral activity against human immunodeficiency virus type 1. J Gen Virol. 2005. 86:2221–2229.
Article68. Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci U S A. 2007. 104:1371–1376.
Article69. Lu G, Reinert JT, Pitha-Rowe I, Okumura A, Kellum M, Knobeloch KP, et al. ISG15 enhances the innate antiviral response by inhibition of IRF-3 degradation. Cell Mol Biol (Noisy-legrand). 2006. 52:29–41.