Korean J Radiol.
2009 Jun;10(3):269-276. 10.3348/kjr.2009.10.3.269.
Ethanol Sclerotherapy for the Management of Craniofacial Venous Malformations: the Interim Results
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. somatom@skku.edu
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 3Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- KMID: 1779454
- DOI: http://doi.org/10.3348/kjr.2009.10.3.269
Abstract
OBJECTIVE
We wanted to evaluate the safety and feasibility of ethanol sclerotherapy for treating craniofacial venous malformations (CVMs). MATERIALS AND METHODS: From May 1998 to April 2007, 87 patients (40 men and 47 women; age range, 2-68 years) with CVMs underwent staged ethanol sclerotherapy (range, 1-21 sessions; median number of sessions, 2) by the direct puncture technique. Clinical follow up (range, 0-120 months; mean follow up, 35 months; median follow up, 28 months) was performed for all the patients. Therapeutic outcomes were established by evaluating the clinical outcome of the signs and symptoms in all patients, as well as the degree of devascularization, which was determined on the follow-up imaging, in 71 patients. RESULTS: A total of 305 procedures with the use of ethanol were performed in 87 patients. Follow-up imaging studies were performed for 71 of 87 patients. Twenty-three (32%) of the 71 patients showed excellent outcomes, 37 patients (52%) showed good outcomes and 11 patients (16%) showed poor outcomes. Ethanol sclerotherapy was considered effective for 60 patients. All the minor complications such as bulla (n = 5) healed with only wound dressing and observation. Any major complication such as skin necrosis did not develop. CONCLUSION: Percutaneous ethanol sclerotherapy is an effective, safe treatment for CVMs.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Analgesics/administration & dosage
Anti-Infective Agents, Local/adverse effects/*therapeutic use
Child
Child, Preschool
Craniofacial Abnormalities/*therapy
Ethanol/adverse effects/*therapeutic use
Feasibility Studies
Female
Follow-Up Studies
Humans
Male
Middle Aged
Pain/drug therapy/etiology
Retrospective Studies
Sclerosing Solutions/adverse effects/therapeutic use
Sclerotherapy/adverse effects/*methods
Treatment Outcome
Vascular Malformations/*therapy
Young Adult
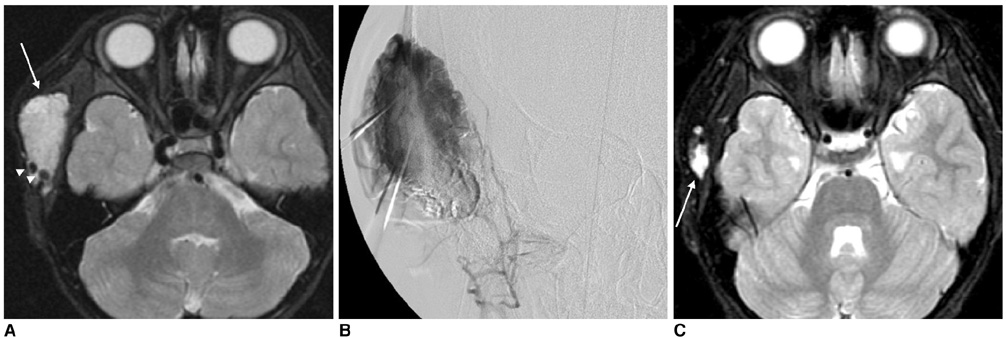
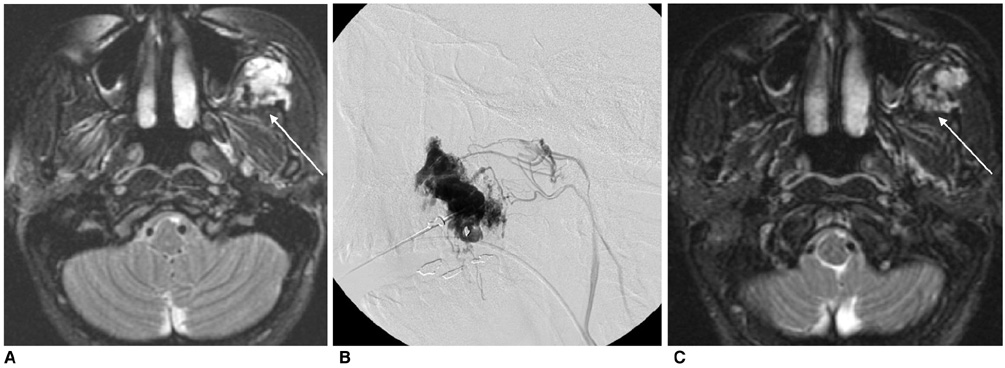
Figure
Cited by 1 articles
-
Sclerotherapy for Venous Malformations of Head and Neck: Systematic Review and Meta-Analysis
Lucio De Maria, Paolo De Sanctis, Karthik Balakrishnan, Megha Tollefson, Waleed Brinjikji
Neurointervention. 2020;15(1):4-17. doi: 10.5469/neuroint.2019.00213.
Reference
-
1. de Lorimier AA. Sclerotherapy for venous malformations. J Pediatr Surg. 1995. 30:188–193.2. Siniluoto TM, Svendsen PA, Wikholm GM, Fogdestam I, Edstrom S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol). Scand J Plast Reconstr Surg Hand Surg. 1997. 31:145–150.3. Yamaki T, Nozaki M, Sasaki K. Color duplex-guided sclerotherapy for the treatment of venous malformations. Dermatol Surg. 2000. 26:323–328.4. Johnson PL, Eckard DA, Brecheisen MA, Girod DA, Tsue TT. Percutaneous ethanol sclerotherapy of venous malformations of the tongue. AJNR Am J Neuroradiol. 2002. 23:779–782.5. Lee BB, Kim DI, Huh S, Kim HH, Choo IW, Byun HS, et al. New experiences with absolute ethanol sclerotherapy in the management of a complex form of congenital venous malformation. J Vasc Surg. 2001. 33:764–772.6. Fishman SJ, Mulliken JB. Vascular anomalies. A primer for pediatricians. Pediatr Clin North Am. 1998. 45:1455–1477.7. Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000. 37:517–584.8. Lee CH, Chen SG. Direct percutaneous ethanol instillation for treatment of venous malformation in the face and neck. Br J Plast Surg. 2005. 58:1073–1078.9. Lee BB. Advanced management of congenital vascular malformations (CVM). Int Angiol. 2002. 21:209–213.10. Lee BB. New approaches to the treatment of congenital vascular malformations (CVMs)-a single centre experience. Eur J Vasc Endovasc Surg. 2005. 30:184–197.11. Lee BB, Do YS, Byun HS, Choo IW, Kim DI, Huh SH. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg. 2003. 37:533–538.12. Rautio R, Laranne J, Kahara V, Saarinen J, Keski-Nisula L. Long-term results and quality of life after endovascular treatment of venous malformations in the face and neck. Acta Radiol. 2004. 45:738–745.13. Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999. 104:1–11.14. Lee CH, Chen SG. Direct percutaneous ethanol sclerotherapy for treatment of a recurrent venous malformation in the periorbital region. ANZ J Surg. 2004. 74:1126–1127.15. Lewin JS, Merkle EM, Duerk JL, Tarr RW. Low-flow vascular malformations in the head and neck: safety and feasibility of MR imaging-guided percutaneous sclerotherapy-preliminary experience with 14 procedures in three patients. Radiology. 1999. 211:566–570.16. Pappas DC Jr, Persky MS, Berenstein A. Evaluation and treatment of head and neck venous vascular malformations. Ear Nose Throat J. 1998. 77:914–916. 918–922.17. Madewell JE, Sweet DE. Resnick D, editor. Tumors and tumor-like lesions in or about joints. Diagnosis of bone and joint disorders. 1995. Philadelphia: WB Saunders;3939–3990.18. Choi YH, Han MH, O-ki K, Cha SH, Chang KH. Craniofacial cavernous venous malformations: percutaneous sclerotherapy with use of ethanolamine oleate. J Vasc Interv Radiol. 2002. 13:475–482.19. Kim KH, Sung MW, Roh JL, Han MH. Sclerotherapy for congenital lesions in the head and neck. Otolaryngol Head Neck Surg. 2004. 131:307–316.20. Mathur NN, Rana I, Bothra R, Dhawan R, Kathuria G, Pradhan T. Bleomycin sclerotherapy in congenital lymphatic and vascular malformations of head and neck. Int J Pediatr Otorhinolaryngol. 2005. 69:75–80.21. Rimon U, Garniek A, Galili Y, Golan G, Bensaid P, Morag B. Ethanol sclerotherapy of peripheral venous malformations. Eur J Radiol. 2004. 52:283–287.22. Yakes WF, Luethke JM, Parker SH, Stavros AT, Rak KM, Hopper KD, et al. Ethanol embolization of vascular malformations. Radiographics. 1990. 10:787–796.23. Dubois JM, Sebag GH, De Prost Y, Teillac D, Chretien B, Brunelle FO. Soft-tissue venous malformations in children: percutaneous sclerotherapy with Ethibloc. Radiology. 1991. 180:195–198.24. O'Donovan JC, Donaldson JS, Morello FP, Pensler JM, Vogelzang RL, Bauer B. Symptomatic hemangiomas and venous malformations in infants, children, and young adults: treatment with percutaneous injection of sodium tetradecyl sulfate. AJR Am J Roentgenol. 1997. 169:723–729.25. Suh JS, Shin KH, Na JB, Won JY, Hahn SB. Venous malformations: sclerotherapy with a mixture of ethanol and lipiodol. Cardiovasc Intervent Radiol. 1997. 20:268–273.26. Mason KP, Michna E, Zurakowski D, Koka BV, Burrows PE. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. 2000. 217:127–132.27. Troughton AH, Paxton RM. Direct puncture venography in subcutaneous cavernous haemangiomas. Clin Radiol. 1992. 45:250–253.28. Behnia R. Systemic effects of absolute alcohol embolization in a patient with a congenital arteriovenous malformation of the lower extremity. Anesth Analg. 1995. 80:415–417.29. Garel L, Mareschal JL, Gagnadoux MF, Pariente D, Guilbert M, Sauvegrain J. Fatal outcome after ethanol renal ablation in child with end-stage kidneys. AJR Am J Roentgenol. 1986. 146:593–594.30. Gelczer RK, Charboneau JW, Hussain S, Brown DL. Complications of percutaneous ethanol ablation. J Ultrasound Med. 1998. 17:531–533.31. Teasdale C, Kirk D, Jeans WD, Penry JB, Tribe CT, Slade N. Arterial embolisation in renal carcinoma: a useful procedure? Br J Urol. 1982. 54:616–619.32. Villavicencio JL. Primum non nocere: Is it always true? The use of absolute ethanol in the management of congenital vascular malformations. J Vasc Surg. 2001. 33:904–906.33. Yakes WF, Haas DK, Parker SH, Gibson MD, Hopper KD, Mulligan JS, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989. 170:1059–1066.34. Yakes WF, Rossi P, Odink H. How I do it. Arteriovenous malformation management. Cardiovasc Intervent Radiol. 1996. 19:65–71.35. Hammer FD, Boon LM, Mathurin P, Vanwijck RR. Ethanol sclerotherapy of venous malformations: evaluation of systemic ethanol contamination. J Vasc Interv Radiol. 2001. 12:595–600.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Venous Malformation of the Pyriform Sinus Treated with Ethanol Sclerotherapy
- Clicically improved venous malformation by sclerotherapy
- Transvaginal Direct Puncture and Ethanol Sclerotherapy for Cervicovaginal Venous Malformations: A Case Report and Literature Review
- Anesthetic Management of Pulmonary Arterial Pressure Change during the Ethanol Sclerotherapy of Peripheral Arteriovenous Malformations
- Cardiovascular collapse due to right heart failure following ethanol sclerotherapy: a case report