Korean J Radiol.
2007 Jun;8(3):216-224. 10.3348/kjr.2007.8.3.216.
FDG-PET for Evaluating the Antitumor Effect of Intraarterial 3-Bromopyruvate Administration in a Rabbit VX2 Liver Tumor Model
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Institute of Radiation Medicine, Seoul National University Medical Research Center and Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. chungjw@radcom.sn
- 2Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Biomedical Research Center, Korea Institute of Science and Technology, Seoul, Korea.
- 5Department of Veterinary Radiology, Chonbuk National University College of Veterinary Medicine, Chonbuk, Korea.
- KMID: 1779437
- DOI: http://doi.org/10.3348/kjr.2007.8.3.216
Abstract
OBJECTIVE
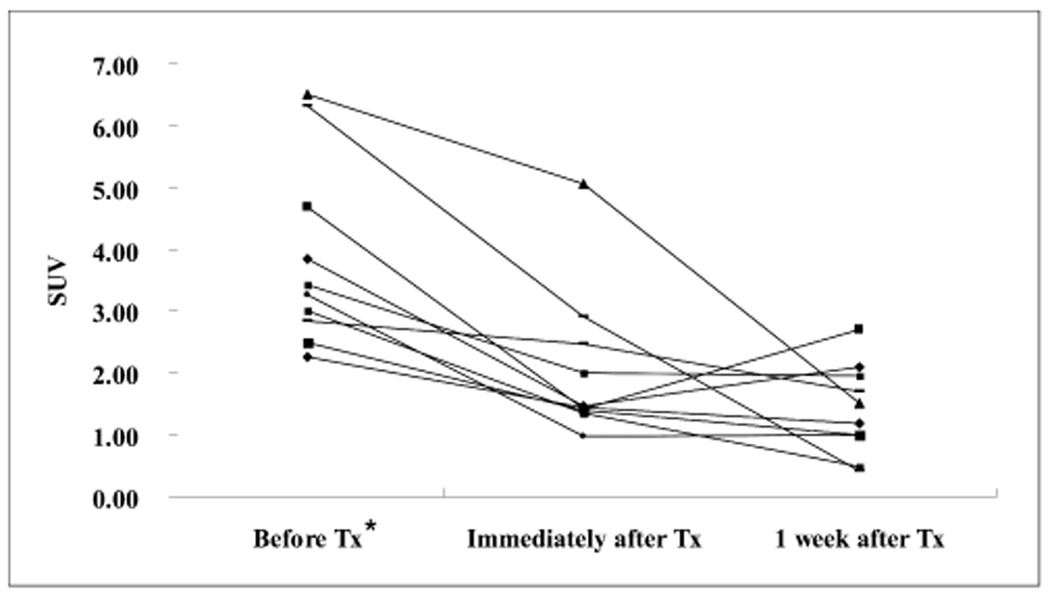
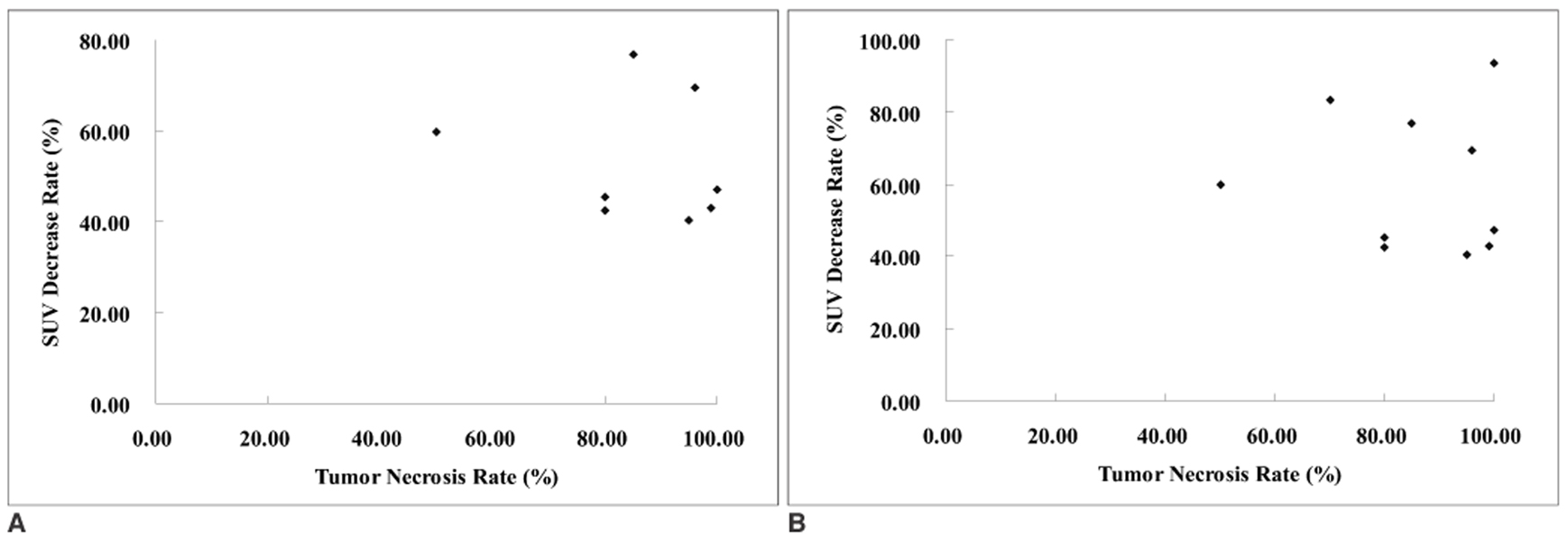
We wanted to investigate the feasibility of using FDG-PET for evaluating the antitumor effect of intraarterial administration of a hexokinase II inhibitor, 3-bromopyruvate (3-BrPA), in a rabbit VX2 liver tumor model. MATERIALS AND METHODS: VX2 carcinoma was grown in the livers of ten rabbits. Two weeks later, liver CT was performed to confirm appropriate tumor growth for the experiment. After tumor volume-matched grouping of the rabbits, transcatheter intraarterial administration of 3-BrPA was performed (1 mM and 5 mM in five animals each, respectively). FDG-PET scan was performed the day before, immediately after and a week after 3-BrPA administration. FDG uptake was semiquantified by measuring the standardized uptake value (SUV). A week after treatment, the experimental animals were sacrificed and the necrosis rates of the tumors were calculated based on the histopathology. RESULTS: The SUV of the VX2 tumors before treatment (3.87+/-1.51[mean+/-SD]) was significantly higher than that of nontumorous liver parenchyma (1.72+/-0.34) (p < 0.0001, Mann-Whitney U test). The SUV was significantly decreased immediately after 3-BrPA administration (2.05+/-1.21) (p = 0.002, Wilcoxon signed rank test). On the one-week follow up PET scan, the FDG uptake remained significantly lower (SUV 1.41+/-0.73) than that before treatment (p = 0.002), although three out of ten animals showed a slightly increasing tendency for the FDG uptake. The tumor necrosis rate ranged from 50.00% to 99.90% (85.48%+/-15.87). There was no significant correlation between the SUV or the SUV decrease rate and the tumor necrosis rate in that range. CONCLUSION: Even though FDG-PET cannot exactly reflect the tumor necrosis rate, FDG-PET is a useful modality for the early assessment of the antitumor effect of intraarterial administration of 3-BrPA in VX2 liver tumor.
MeSH Terms
-
Animals
Disease Models, Animal
Enzyme Inhibitors/*pharmacology
Feasibility Studies
Fluorodeoxyglucose F18
Infusions, Intra-Arterial
Injections, Intra-Arterial
Liver Neoplasms, Experimental/*drug therapy/pathology/*radionuclide imaging
Necrosis
*Positron-Emission Tomography
Pyruvate Dehydrogenase Complex/antagonists & inhibitors
Pyruvates/*pharmacology
Rabbits
Radiopharmaceuticals
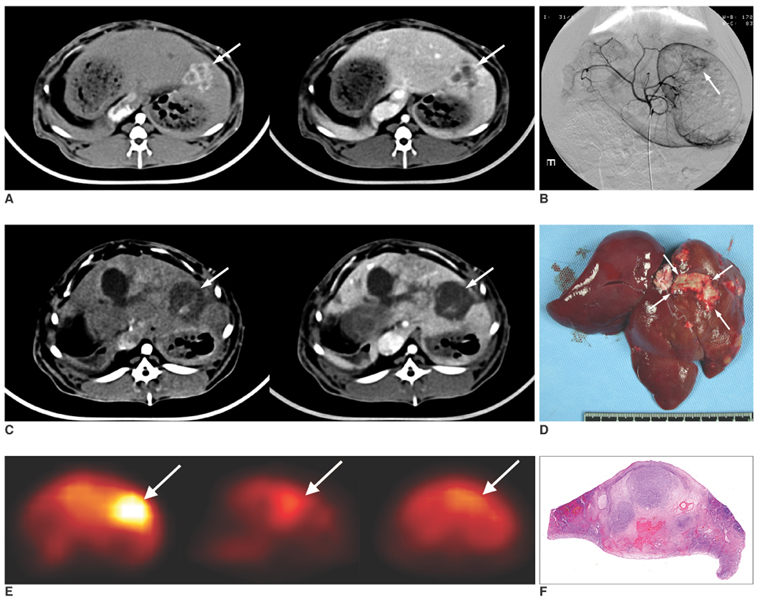
Figure
Cited by 1 articles
-
The Antitumor Effect and Hepatotoxicity of a Hexokinase II Inhibitor 3-Bromopyruvate:
In Vivo Investigation of Intraarterial Administration in a Rabbit VX2 Hepatoma Model
Hwan Jun Jae, Jin Wook Chung, Hee Sun Park, Min Jong Lee, Ki Chang Lee, Hyo-Cheol Kim, Jung Hwan Yoon, Hesson Chung, Jae Hyung Park
Korean J Radiol. 2009;10(6):596-603. doi: 10.3348/kjr.2009.10.6.596.
Reference
-
1. Di Bisceglie AM, Rustgi VK, Hoofnagle JH, Dusheiko GM, Lotze MT. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988. 108:390–401.2. Rustgi VK. Epidemiology of hepatocellular carcinoma. Gastroenterol Clin North Am. 1987. 16:545–551.3. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985. 56:918–928.4. Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002. 13:S211–S221.5. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983. 148:397–401.6. Bruix J. Treatment of hepatocellular carcinoma. Hepatology. 1997. 25:259–262.7. Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978. 22:190–274.8. Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988. 263:17422–17428.9. Greiner EF, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994. 269:31484–31490.10. Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001. 276:43407–43412.11. Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972. 32:2007–2016.12. Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta. 2002. 1555:14–20.13. Shinohara Y, Ichihara J, Terada H. Remarkably enhanced expression of the type II hexokinase in rat hepatoma cell line AH130. FEBS Lett. 1991. 291:55–57.14. Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells. Isolation, sequence, and activity of the promoter for type II hexokinase. J Biol Chem. 1995. 270:16918–16925.15. Nakashima RA, Paggi MG, Scott LJ, Pedersen PL. Purification and characterization of a bindable form of mitochondrial bound hexokinase from the highly glycolytic AS-30D rat hepatoma cell line. Cancer Res. 1988. 48:913–919.16. Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001. 173:83–91.17. Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002. 62:3909–3913.18. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978. 19:1154–1161.19. Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004. 24:523–543.20. Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004. 231:305–332.21. Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004. 324:269–275.22. Okada M, Kudo S, Miyazaki O, Saino T, Ekimoto H, Iguchi H, et al. Antitumoral efficacy and pharmacokinetic properties of pirarubicin upon hepatic intra-arterial injection in the rabbit V x2 tumour model. Br J Cancer. 1995. 71:518–524.23. Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005. 42:358–364.24. Som P, Atkins HL, Bandoypadhyay D, Fowler JS, MacGregor RR, Matsui K, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980. 21:670–675.25. Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991. 32:623–648.26. Oya N, Nagata Y, Ishigaki T, Abe M, Tamaki N, Magata Y, et al. Evaluation of experimental liver tumors using fluorine-18-2-fluoro-2-deoxy-D-glucose PET. J Nucl Med. 1993. 34:2124–2129.27. Messa C, Choi Y, Hoh CK, Jacobs EL, Glaspy JA, Rege S, et al. Quantification of glucose utilization in liver metastases: parametric imaging of FDG uptake with PET. J Comput Assist Tomogr. 1992. 16:684–689.28. Nagata Y, Yamamoto K, Hiraoka M, Abe M, Takahashi M, Akuta K, et al. Monitoring liver tumor therapy with [18F]FDG positron emission tomography. J Comput Assist Tomogr. 1990. 14:370–374.29. Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, et al. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992. 33:333–339.30. Torizuka T, Tamaki N, Inokuma T, Magata Y, Yonekura Y, Tanaka A, et al. Value of fluorine-18-FDG-PET to monitor hepatocellular carcinoma after interventional therapy. J Nucl Med. 1994. 35:1965–1969.31. Oya N, Nagata Y, Tamaki N, Takagi T, Murata R, Magata Y, et al. FDG-PET evaluation of therapeutic effects on VX2 liver tumor. J Nucl Med. 1996. 37:296–302.32. Spaepen K, Stroobants S, Dupont P, Bormans G, Balzarini J, Verhoef G, et al. [(18)F]FDG PET monitoring of tumour response to chemotherapy: does [(18)F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging. 2003. 30:682–688.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Antitumor Effect and Hepatotoxicity of a Hexokinase II Inhibitor 3-Bromopyruvate: In Vivo Investigation of Intraarterial Administration in a Rabbit VX2 Hepatoma Model
- Development of Rabbit Brain Tumor Model Using VX2 Cells and Verification with the MRI in Neuroradiologic Research
- Application of Simultaneous 18F-FDG PET/MRI for Evaluating Residual Lesion in Pyogenic Spine Infection:a Case Report
- Evaluation of Angiogenesis of Hepatic VX2 Carcinoma and Abscess in the Rabbit Liver with Perfusion Computed Tomography
- Anti-tumoral Effect of Recombinant Vaccinia Virus through US Guided Injection in a Rabbit Model of Hepatic VX2 Carcinoma