J Korean Med Sci.
2010 Nov;25(11):1601-1608. 10.3346/jkms.2010.25.11.1601.
Clinical Benefit of Low Molecular Weight Heparin for ST-segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention with Glycoprotein IIb/IIIa Inhibitor
- Affiliations
-
- 1Cardiovascular Center, Seoul St. Mary's Hospital, Seoul, Korea.
- 2Cardiovascular Center, Chonnam National University Hospital, Gwangju, Korea. myungho@chollian.net
- 3Cardiovascular Center, Kyungpook National University, Daegu, Korea.
- 4Cardiovascular Center, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 5Cardiovascular Center, Pusan National University Hospital, Busan, Korea.
- 6Cardiovascular Center, Yeungnam University Hostpial, Daegu, Korea.
- 7Cardiovascular Center, Chungnam National University Hostpial, Daejon, Korea.
- 8Cardiovascular Center, Chonbuk National University Hospital, Jeonju, Korea.
- 9Cardiovascular Center, Jeonju Presbyterian Medical Center, Jeonju, Korea.
- 10Cardiovascular Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 11Cardiovascular Center, Chungbuk National University, Cheongju, Korea.
- 12Cardiovascular Center, Konyang University, Daejon, Korea.
- 13Cardiovascular Center, Korea University Guro Hospital, Seoul, Korea.
- 14Cardiovascular Center, Kyunghee University Hospital, Seoul, Korea.
- 15Cardiovascular Center, Yonsei University Hospital, Seoul, Korea.
- 16Cardiovascular Center, Wonju University Hospital, Wonju, Korea.
- 17Cardiovascular Center, Ulsan University Asan Medical Center, Seoul, Korea.
- KMID: 1779230
- DOI: http://doi.org/10.3346/jkms.2010.25.11.1601
Abstract
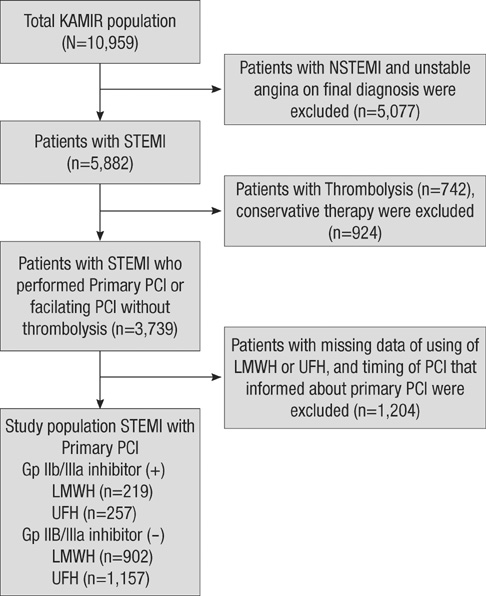
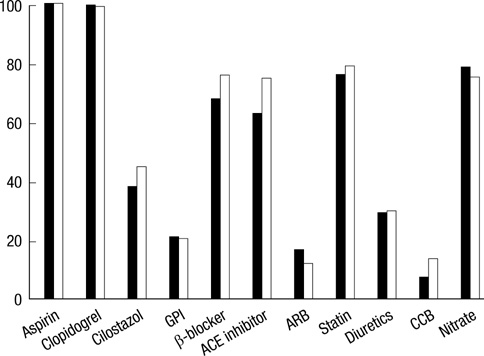
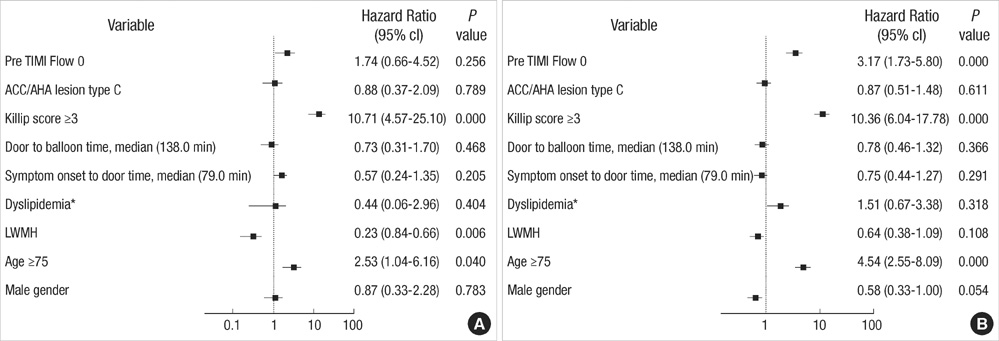
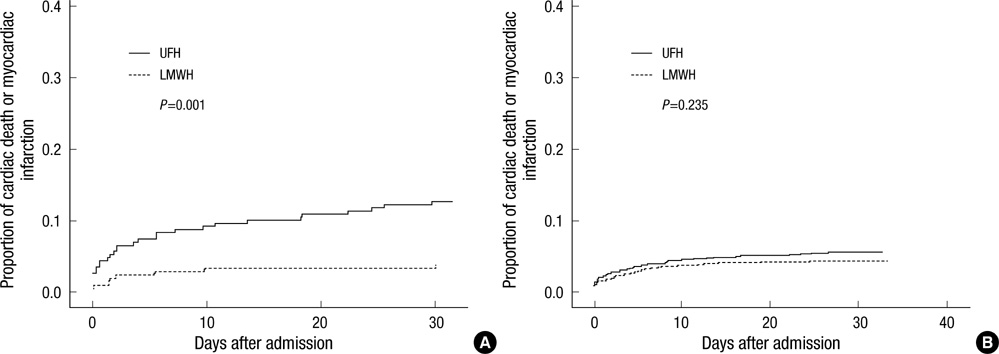
- The efficacy of low molecular weight heparin (LMWH) with low dose unfractionated heparin (UFH) during percutaneous coronary intervention (PCI) with or without glycoprotein (Gp) IIb/IIIa inhibitor compared to UFH with or without Gp IIb/IIIa inhibitor has not been elucidated. Between October 2005 and July 2007, 2,535 patients with ST elevation acute myocardial infarction (STEMI) undergoing PCI in the Korean Acute Myocardial Infarction Registry (KAMIR) were assigned to either of two groups: a group with Gp IIb/IIIa inhibitor (n=476) or a group without Gp IIb/IIIa inhibitor (n=2,059). These groups were further subdivided according to the use of LMWH with low dose UFH (n=219) or UFH alone (n=257). The primary end points were cardiac death or myocardial infarction during the 30 days after the registration. The primary end point occurred in 4.1% (9/219) of patients managed with LMWH during PCI and Gp IIb/IIIa inhibitor and 10.8% (28/257) of patients managed with UFH and Gp IIb/IIIa inhibitor (odds ratio [OR], 0.290; 95% confidence interval [CI], 0.132-0.634; P=0.006). Thrombolysis In Myocardial Infarction (TIMI) with major bleeding was observed in LMHW and UFH with Gp IIb/IIIa inhibitor (1/219 [0.5%] vs 1/257 [0.4%], P=1.00). For patients with STEMI managed with a primary PCI and Gp IIb/IIIa inhibitor, LMWH is more beneficial than UFH.
Keyword
MeSH Terms
-
Acute Disease
Aged
Drug Therapy, Combination
Female
Hemorrhage
Heparin/*therapeutic use
Heparin, Low-Molecular-Weight/*therapeutic use
Humans
Male
Middle Aged
Multivariate Analysis
Myocardial Infarction/epidemiology/mortality/*therapy
Myocardial Revascularization
Odds Ratio
Platelet Glycoprotein GPIIb-IIIa Complex/*antagonists & inhibitors/metabolism
Prognosis
Registries
Figure
Reference
-
1. Collet JP, Montalescot G, Agnelli G, Van de Werf F, Gurfinkel EP, López-Sendón J, Laufenberg CV, Klutman M, Gowda N, Gulba D. GRACE Investigators. Non-ST-segment elevation acute coronary syndrome in patients with renal dysfunction: benefit of low-molecular-weight heparin alone or with glycoprotein IIb/IIIa inhibitors on outcomes. The Global Registry of Acute Coronary Events. Eur Heart J. 2005. 26:2285–2293.
Article2. Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H. SYNERGY Trial Investigators. Enoxaparin vs. unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004. 292:45–54.
Article3. White HD, Kleiman NS, Mahaffey KW, Lokhnygina Y, Pieper KS, Chiswell K, Cohen M, Harrington RA, Chew DP, Petersen JL, Berdan LG, Aylward PE, Nessel CC, Ferguson JJ 3rd, Califf RM. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial. Am Heart J. 2006. 152:1042–1050.
Article4. Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomized trial in acute myocardial infarction. Lancet. 2001. 358:605–613.5. Wallentin L, Goldstein P, Armstrong PW, Granger CB, Adgey AA, Arntz HR, Bogaerts K, Danays T, Lindahl B, Mäkijärvi M, Verheugt F, Van de Werf F. Efficacy and safety of tenecteplase in combination with the low-molecular-weight heparin enoxaparin or unfractionated heparin in the prehospital setting: the Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 PLUS randomized trial in acute myocardial infarction. Circulation. 2003. 108:135–142.6. Antman EM, Morrow DA, McCabe CH, Murphy SA, Ruda M, Sadowski Z, Budaj A, López-Sendón JL, Guneri S, Jiang F, White HD, Fox KA, Braunwald E. ExTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med. 2006. 354:1477–1488.
Article7. Montalescot G, White HD, Gallo R, Cohen M, Steg PG, Aylward PE, Bode C, Chiariello M, King SB 3rd, Harrington RA, Desmet WJ, Macaya C, Steinhubl SR. STEEPLE Investigators. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med. 2006. 355:1006–1017.
Article8. Montalescot G, Ellis SG, de Belder MA, Janssens L, Katz O, Pluta W, Ecollan P, Tendera M, van Boven AJ, Widimsky P, Andersen HR, Betriu A, Armstrong P, Brodie BR, Herrmann HC, Neumann FJ, Effron MB, Lu J, Barnathan ES, Topol EJ. Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events Investigators. Enoxaparin in primary and facilitated percutaneous coronary intervention A formal prospective non-randomized substudy of the FINESSE trial (Facilitated INtervention with Enhanced Reperfusion Speed to Stop Events). JACC Cardiovasc Interv. 2010. 3:203–212.9. Klinkhardt U, Graff J, Westrup D, Kirchmaier CM, Esslinger HU, Breddin HK, Harder S. Pharmacodynamic characterization of the interaction between abciximab or tirofiban with unfractionated or a low molecular weight heparin in healthy subjects. Br J Clin Pharmacol. 2001. 52:297–305.
Article10. Xiao Z, Theroux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low molecular-weight heparin and with a direct thrombin inhibitor. Circulation. 1998. 97:251–256.11. Westwick J, Scully MF, Poll C, Kakkar W. Comparison of low molecular weight heparin and unfractionated heparin on activation of human platelets in vitro. Thromb Res. 1986. 42:435–447.12. Deliargyris EN, Upadhya B, Melton LG, Thompson C, Fisher M, Gabriel DA, Dehmer GJ, Sane DC. Unfractionated heparin reduces the anti-platelet effects of abciximab but not eptifibatide during PCI. Clin Appl Thromb Hemost. 2006. 12:458–464.
Article13. Lee SR, Jeong MH, Ahn YK, Chae SC, Hur SH, Kim YJ, Seong IW, Chae JK, Hong TJ, Rhew JY, Cho MC, Bae JH, Rha SW, Kim CJ, Jang YS, Park SJ. Korea Acute Myocardial Infarction Registry Investigators. Clinical safety of drug-eluting stents in the Korea Acute Myocardial Infarction Registry. Circ J. 2008. 72:392–398.
Article14. Lee SH, Park JS, Kim W, Shin DG, Kim YJ, Kim DS, Choi DJ, Han KR, Kim CJ, Cho MC, Chae SC, Jeong MH. Korean Acute Myocardial Infarction Registry Investigators. Impact of body mass index and waist-to-hip ratio on clinical outcomes in patients with ST-segment elevation acute myocardial infarction (from the Korean Acute Myocardial Infarction Registry). Am J Cardiol. 2008. 102:957–965.
Article15. Song YB, Hahn JY, Gwon HC, Kim JH, Lee SH, Jeong MH. KAMIR investigators. The impact of initial treatment delay using primary angioplasty on mortality among patients with acute myocardial infarction: from the Korea Acute Myocardial Infarction Registry. J Korean Med Sci. 2008. 23:357–364.
Article16. Lee JH, Park HS, Chae SC, Cho Y, Yang DH, Jeong MH, Kim YJ, Kim KS, Hur SH, Seong IW, Hong TJ, Cho MC, Kim CJ, Jun JE, Park WH. Korea Acute Myocardial Infarction Registry Investigators. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Am J Cardiol. 2009. 104:182–189.
Article17. Kwon TG, Bae JH, Jeong MH, Kim YJ, Hur SH, Seong IW, Cho MC, Seung KB, Jang YS, Park SJ. Korea Acute Myocardial Infarction Registry Investigators. N-terminal pro-B-type natriuretic peptide is associated with adverse short-term clinical outcomes in patients with acute ST-elevation myocardial infarction underwent primary percutaneous coronary intervention. Int J Cardiol. 2009. 133:173–178.
Article18. Li YJ, Rha SW, Chen KY, Poddar KL, Jin Z, Minami Y, Wang L, Dang Q, Li GP, Ramasamy S, Park JY, Choi CU, Kim JW, Kim EJ, Park CG, Seo HS, Oh DJ, Jeong MH, Ahn YK, Hong TJ, Park JS, Kim YJ, Hur SH, Seong IW, Chae JK, Cho MC, Bae JH, Choi DH, Jang YS, Chae IH, Kim HS, Kim CJ, Yoon JH, Ahn TH, Tahk SJ, Chung WS, Seung KB, Park SJ. other Korea Acute Myocardial infarction Registry Investigators. Low-molecular-weight heparin versus unfractionated heparin in acute ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention with drug-eluting stents. Am Heart J. 2010. 159:684–690.
Article19. Thomson C, Forbes CD, Prentice CR. The potentiation of platelet aggregation and adhesion by heparin in vitro and in vivo. Clin Sci Mol Med. 1973. 45:485–494.
Article20. Eika C. On the mechanism of platelet aggregation induced by heparin, protamine and polybrene. Scand J Haematol. 1972. 9:248–257.
Article21. Steinhubl SR, Talley JD, Braden GA, Tcheng JE, Casterella PJ, Moliterno DJ, Navetta FI, Berger PB, Popma JJ, Dangas G, Gallo R, Sane DC, Saucedo JF, Jia G, Lincoff AM, Theroux P, Holmes DR, Teirstein PS, Kereiakes DJ. Point-of-Care measured platelet inhibition correlates with a reduced risk of an adverse cardiac even after percutaneous coronary intervention. Results of the GOLD (AU-assessing Ultegra) Multicenter Study. Circulation. 2001. 103:2572–2578.22. The IMPACT II Investigators. Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet. 1997. 349:1422–1428.23. Ruff CT, Wiviott SD, Morrow DA, Mohanavelu S, Murphy SA, Antman EM, Braunwald E. ExTRACT-TIMI 25 Investigators. TIMI risk index and the benefit of enoxaparin in patients with ST-elevation myocardial infarction. Am J Med. 2007. 120:993–998.
Article24. Murphy SA, Gibson CM, Morrow DA, Van de Werf F, Menown IB, Goodman SG, Mahaffey KW, Cohen M, McCabe CH, Antman EM, Braunwald E. Efficacy and safety of the low-molecular weight heparin enoxaparin compared with unfractionated heparin across the acute coronary syndrome spectrum: a meta-analysis. Eur Heart J. 2007. 28:2077–2086.
Article25. Montalescot G, Collet JP, Lison L, Choussat R, Ankri A, Vicaut E, Perlemuter K, Philippe F, Drobinski G, Thomas D. Effects of various anticoagulant treatments on von Willebrand factor release in unstable angina. J Am Coll Cardiol. 2000. 36:110–114.
Article26. Simoons ML. GUSTO IV-ACS Investigators. Effect of glycoprotein Ilb/Illa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularization: the GUSTO IV-ACS randomised trial. Lancet. 2001. 357:1915–1924.27. Burgess JK, Chong BH. The platelet proaggregating and potentiating effects of unfractionated heparin, low molecular weight heparin and heparinoid in intensive care patients and health controls. Eur J Haematol. 1997. 58:279–285.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Cerebral Infarction Following Intravenous Glycoprotein IIb/IIIa Inhibitor for Acute Myocardial Infarction
- Percutaneous Coronary Intervention for Coronary Artery Disease
- The Long-Term Clinical Outcomes of Low Molecular Weight Heparin Combined with Platelet Glycoprotein IIb/IIIa Inhibitor in Patients with Acute Coronary Syndrome
- Consecutive Multivessel Myocardial Infarction during Primary Percutaneous Coronary Intervention
- Clinical outcome in elderly patients older than 70 years with acute myocardial infarction after use of platelet glycoprotein IIb/IIIa receptor blocker during percutaneous coronary intervention: Comparison with those younger than 70 years