J Korean Med Sci.
2009 Aug;24(4):660-667. 10.3346/jkms.2009.24.4.660.
High-dose Chemotherapy and Autologous Stem Cell Rescue in Patients with High-risk Stage 3 Neuroblastoma: 10-Year Experience at a Single Center
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kwsped@skku.edu
- 2Department of Pediatric Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1779200
- DOI: http://doi.org/10.3346/jkms.2009.24.4.660
Abstract
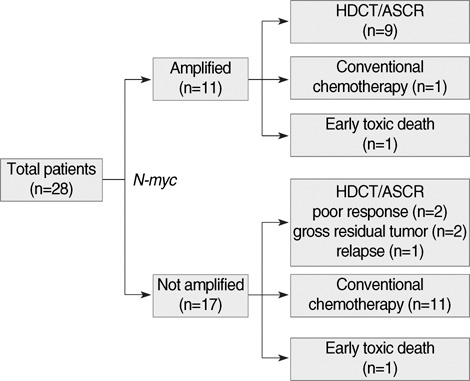
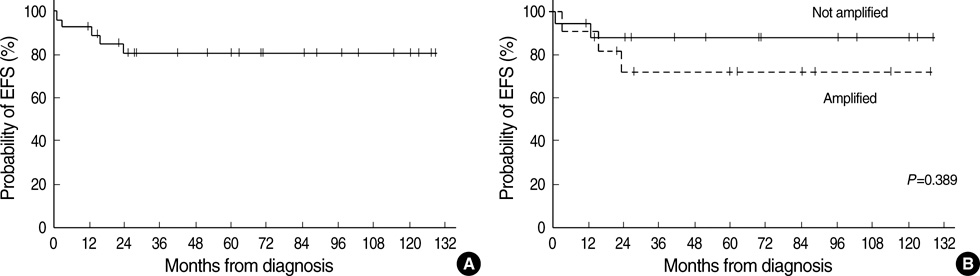
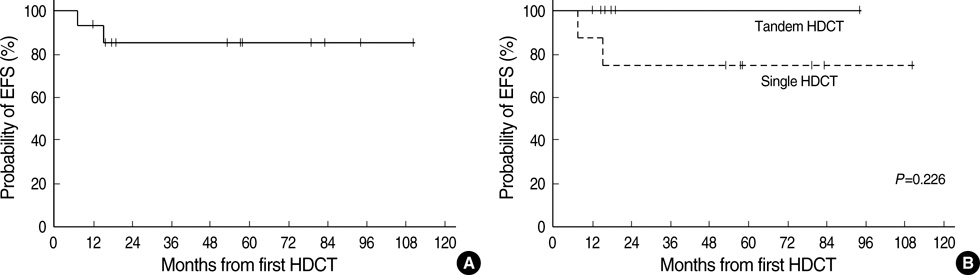
- High-dose chemotherapy and autologous stem cell rescue (HDCT/ASCR) was applied to improve the prognosis of patients with high-risk stage 3 neuroblastoma. From January 1997 to December 2006, 28 patients were newly diagnosed as stage 3 neuroblastoma. Nine of 11 patients with N-myc amplification and 5 of 17 patients without N-myc amplification (poor response in 2 patients, persistent residual tumor in 2 and relapse in 1) underwent single or tandem HDCT/ASCR. Patients without high-risk features received conventional treatment modalities only. While 8 of 9 patients underwent single HDCT/ASCR and the remaining one patient underwent tandem HDCT/ASCR during the early study period, all 5 patients underwent tandem HDCT/ASCR during the late period. Toxicities associated with HDCT/ASCR were tolerable and there was no treatment-related mortality. While the tumor relapsed in two of eight patients in single HDCT/ASCR group, all six patients in tandem HDCT/ASCR group remained relapse free. The 5-yr event-free survival (EFS) from diagnosis, in patients with N-myc amplification, was 71.6+/-14.0%. In addition, 12 of 14 patients who underwent HDCT/ASCR remained event free resulting in an 85.1+/-9.7% 5-yr EFS after the first HDCT/ASCR. The present study demonstrates that HDCT/ASCR may improve the survival of patients with high-risk stage 3 neuroblastoma.
MeSH Terms
-
Adolescent
Adult
Child
Combined Modality Therapy
Female
Granulocyte Colony Stimulating Factor, Recombinant/therapeutic use
Humans
Male
Middle Aged
Neoplasm Staging
Neuroblastoma/drug therapy/mortality/*therapy
*Peripheral Blood Stem Cell Transplantation
Proto-Oncogene Proteins c-myc/analysis/genetics
Survival Rate
Tomography, X-Ray Computed
Transplantation, Autologous
Figure
Reference
-
1. Matthay KK, Perez C, Seeger RC, Brodeur GM, Shimada H, Atkinson JB, Black CT, Gerbing R, Haase GM, Stram DO, Swift P, Lukens JN. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. J Clin Oncol. 1998. 16:1256–1264.
Article2. Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005. 17:7–13.
Article3. Rubie H, Hartmann O, Michon J, Frappaz D, Coze C, Chastagner P, Baranzelli MC, Plantaz D, Avet-Loiseau H, Benard J, Delattre O, Favrot M, Peyroulet MC, Thyss A, Perel Y, Bergeron C, Courbon-Collet B, Vannier JP, Lemerle J, Sommelet D. N-Myc gene amplification is a major prognostic factor in localized neuroblastoma: results of the French NBL 90 study. Neuroblastoma Study Group of the Société Francaise d'Oncologie Pédiatrique. J Clin Oncol. 1997. 15:1171–1182.
Article4. Castel V, Garcia-Miguel P, Canete A, Melero C, Navajas A, Ruiz-Jimenez JI, Navarro S, Badal MD. Prospective evaluation of the International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Response Criteria (INRC) in a multicentre setting. Eur J Cancer. 1999. 35:606–611.
Article5. La Quaglia MP, Kushner BH, Su W, Heller G, Kramer K, Abramson S, Rosen N, Wolden S, Cheung NK. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004. 39:412–417.
Article6. von Schweinitz D, Hero B, Berthold F. The impact of surgical radicality on outcome in childhood neuroblastoma. Eur J Pediatr Surg. 2002. 12:402–409.
Article7. Stram DO, Matthay KK, O'Leary M, Reynolds CP, Haase GM, Atkinson JB, Brodeur GM, Seeger RC. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children's Cancer Group studies. J Clin Oncol. 1996. 14:2417–2426.
Article8. Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999. 341:1165–1173.9. Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R, Michon J, Prichard J, Klingebiel T, Kremens B, Pearson A, Coze C, Paolucci P, Frappaz D, Gadner H, Chauvin F. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998. 16:953–965.
Article10. Cohn SL, Moss TJ, Hoover M, Katzenstein HM, Haut PR, Morgan ER, Green AA, Kletzel M. Treatment of poor-risk neuroblastoma patients with high-dose chemotherapy and autologous peripheral stem cell rescue. Bone Marrow Transplant. 1997. 20:543–551.
Article11. Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, Simon T, Hero B. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomized controlled trial. Lancet Oncol. 2005. 6:649–658.12. Sung KW, Lee SH, Yoo KH, Jung HL, Cho EJ, Koo HH, Lee SK, Kim J, Lim DH, Suh YL, Kim DW. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007. 40:37–45.
Article13. George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, Pulsipher M, Grupp SA, Diller L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006. 24:2891–2896.
Article14. Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, Geissler G, Marymount MH, Liu D, Kalapurakal JA, Shore RM, Bardo DM, Schmoldt J, Rademaker AW, Cohn SL. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002. 20:2284–2292.
Article15. Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993. 11:1466–1477.
Article16. Pession A, Prete A, Locatelli F, Pierinelli S, Pession AL, Maccario R, Magrini E, De Bernardi B, Paolucci P, Paolucci G. Immunotherapy with low-dose recombinant interleukin 2 after high-dose chemotherapy and autologous stem cell transplantation in neuroblastoma. Br J Cancer. 1998. 78:528–533.
Article17. Flandin I, Hartmann O, Michon J, Pinkerton R, Coze C, Stephan JL, Fourquet B, Valteau-Couanet D, Bergeron C, Philip T, Carrie C. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int J Radiat Oncol Biol Phys. 2006. 64:1424–1431.
Article18. Meacham LR, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL, Oeffinger KC. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005. 103:1730–1739.19. Finklestein JZ, Krailo MD, Lenarsky C, Ladisch S, Blair GK, Reynolds CP, Sitarz AL, Hammond GD. 13-cis-retinoic acid (NSC 122758) in the treatment of children with metastatic neuroblastoma unresponsive to conventional chemotherapy: report from the Childrens Cancer Study Group. Med Pediatr Oncol. 1992. 20:307–311.
Article20. Kohler JA, Imeson J, Ellershaw C, Lie SO. A randomized trial of 13-Cis retinoic acid in children with advanced neuroblastoma after high-dose therapy. Br J Cancer. 2000. 83:1124–1127.
Article21. Matthay KK, Reynolds CP. Is there a role for retinoids to treat minimal residual disease in neuroblastoma? Br J Cancer. 2000. 83:1121–1123.
Article22. Shin MY, Ahn KM, Sung KW, Koo HH. Immunotherapy with interleukin-2 after autologous stem cell transplantation in children with high-risk solid tumor. Korean J Hematol Stem Cell Trans. 1999. 4:239–248.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High Dose Chemotherapy and Autologous Peripheral Blood Stem Cell Transplantation in Pediatric Patients with High-risk Neuroblastoma
- A Case of Autologous Cord Blood Stem Cell Transplantationin Stage IV Neuroblastoma
- Triple High Dose Chemotherapy Followed by Autologous Stem Cell Transplantation for Pediatric Neuroblastoma
- Improved Survival in Neuroblastoma by Autologous Peripheral Blood Stem Cell Transplantation: A Single Institution Experience
- Treatment of high-risk neuroblastoma