J Korean Med Sci.
2009 Apr;24(2):262-268. 10.3346/jkms.2009.24.2.262.
Cessation of Gonadotropin-Releasing Hormone Antagonist on Triggering Day: An Alternative Method for Flexible Multiple-Dose Protocol
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea. suhcs@snu.ac.kr
- 2Health Promotion Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Obstetrics and Gynecology, College of Medicine, Seoul National University, Seoul, Korea.
- KMID: 1779128
- DOI: http://doi.org/10.3346/jkms.2009.24.2.262
Abstract
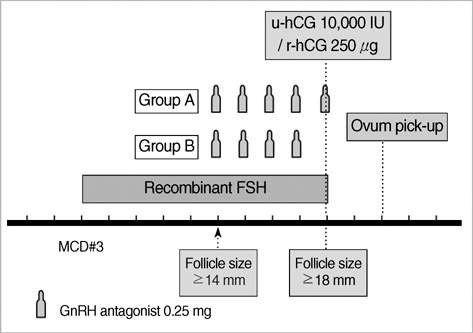
- This study was performed to analyze retrospectively outcomes of stimulated in vitro fertilization (IVF) cycles where the gonadotropin-releasing hormone (GnRH) antagonist was omitted on ovulation triggering day. A total of 92 consecutive IVF cycles were included in 65 women who are undergoing ovarian stimulation with recombinant FSH. A GnRH antagonist, cetrorelix 0.25 mg/day, was started when leading follicle reached 14 mm in diameter until the day of hCG administration (Group A, 66 cycles) or until the day before hCG administration (Group B, 26 cycles). The duration of ovarian stimulation, total dose of gonadotropins, serum estradiol levels on hCG administration day, and the number of oocytes retrieved were not significantly different between the two groups. The total dose of GnRH antagonist was significantly lower in Group B compared to Group A (2.7+/-0.8 vs. 3.2+/-0.9 ampoules). There was no premature luteinization in the subjects. The proportion of mature oocytes (71.4% vs. 61.7%) and fertilization rate of mature (86.3+/-19.7% vs. 71.8+/-31.7%) was significantly higher in Group B. There were no significant differences in embryo quality and clinical pregnancy rates. Our results suggest that cessation of the GnRH antagonist on the day of hCG administration during a flexible multiple-dose protocol could reduce the total dose of GnRH antagonist without compromising IVF results.
Keyword
MeSH Terms
-
Adult
Chorionic Gonadotropin/administration & dosage
Drug Administration Schedule
Estradiol/blood
Female
Fertilization in Vitro
Follicle Stimulating Hormone/administration & dosage/blood
Gonadotropin-Releasing Hormone/*antagonists & inhibitors
Hormone Antagonists/*administration & dosage
Humans
Ovulation Induction/*methods
Recombinant Proteins/therapeutic use
Retrospective Studies
Figure
Reference
-
1. Diedrich K, Diedrich C, Santos E, Zoll C, al-Hasani S, Reissmann T, Krebs D, Klingmuller D. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. 1994. 9:788–791.
Article2. Olivennes F. The use of gonadotropin-releasing hormone antagonist in ovarian stimulation. Clin Obstet Gynecol. 2006. 49:12–22.
Article3. Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a Cochrane review. Hum Reprod. 2002. 17:874–885.
Article4. The ganirelix dose-finding study group. A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod. 1998. 13:3023–3031.5. Al-Inany H, Aboulghar MA, Mansour RT, Serour GI. Ovulation induction in the new millennium: recombinant follicle-stimulating hormone versus human menopausal gonadotropin. Gynecol Endocrinol. 2005. 20:161–169.
Article6. Olivennes F, Belaisch-Allart J, Emperaire JC, Dechaud H, Alvarez S, Moreau L, Nicollet B, Zorn JR, Bouchard P, Frydman R. Prospective, randomized, controlled study of in vitro fertilization-embryo transfer with a single dose of a luteinizing hormone-releasing hormone (LH-RH) antagonist (cetrorelix) or a depot formula of an LH-RH agonist (triptorelin). Fertil Steril. 2000. 73:314–320.
Article7. Roulier R, Chabert-Orsini V, Sitri MC, Barry B, Terriou P. Depot GnRH agonist versus the single dose GnRH antagonist regimen (cetrorelix, 3 mg) in patients undergoing assisted reproduction treatment. Reprod Biomed Online. 2003. 7:185–189.
Article8. Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006. 3:CD001750.
Article9. Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, Griesinger G. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum Reprod Update. 2006. 12:651–671.
Article10. Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007. 22:2805–2813.
Article11. Griesinger G, Felberbaum R, Diedrich K. GnRH antagonists in ovarian stimulation: a treatment regimen of clinicians' second choice? Data from the German national IVF registry. Hum Reprod. 2005. 20:2373–2375.
Article12. Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Hum Reprod. 2006. 21:352–357.
Article13. Kolibianakis EM, Albano C, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Initiation of gonadotropin-releasing hormone antagonist on day 1 as compared to day 6 of stimulation: effect on hormonal levels and follicular development in in vitro fertilization cycles. J Clin Endocrinol Metab. 2003. 88:5632–5637.14. Frankfurter D, Dayal M, Dubey A, Peak D, Gindoff P. Novel follicular-phase gonadotropin-releasing hormone antagonist stimulation protocol for in vitro fertilization in the poor responder. Fertil Steril. 2007. 88:1442–1445.
Article15. Griesinger G, Dawson A, Schultze-Mosgau A, Finas D, Diedrich K, Felberbaum R. Assessment of luteinizing hormone level in the gonadotropin-releasing hormone antagonist protocol. Fertil Steril. 2006. 85:791–793.
Article16. Allegra A, Marino A, Coffaro F, Scaglione P, Sammartano F, Rizza G, Volpes A. GnRH antagonist-induced inhibition of the premature LH surge increases pregnancy rates in IUI-stimulated cycles. A prospective randomized trial. Hum Reprod. 2007. 22:101–108.
Article17. Fauser BC, Devroey P. Why is the clinical acceptance of gonadotropin-releasing hormone antagonist cotreatment during ovarian hyperstimulation for in vitro fertilization so slow? Fertil Steril. 2005. 83:1607–1611.
Article18. Brown JB. Pituitary control of ovarian function-concepts derived from gonadotrophin therapy. Aust N Z J Obstet Gynaecol. 1978. 18:46–54.19. Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994. 9:188–191.
Article20. Hernandez ER. Embryo implantation and GnRH antagonists: embryo implantation: the Rubicon for GnRH antagonists. Hum Reprod. 2000. 15:1211–1216.21. Ortmann O, Diedrich K. Pituitary and extrapituitary actions of gonadotrophin-releasing hormone and its analogues. Hum Reprod. 1999. 14:Suppl 1. 194–206.
Article22. Grundker C, Gunthert AR, Millar RP, Emons G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J Clin Endocrinol Metab. 2002. 87:1427–1430.
Article23. Peng C, Fan NC, Ligier M, Vaananen J, Leung PC. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994. 135:1740–1746.
Article24. Ikemoto T, Park MK. Comparative analysis of the pituitary and ovarian GnRH systems in the leopard gecko: signaling crosstalk between multiple receptor subtypes in ovarian follicles. J Mol Endocrinol. 2007. 38:289–304.
Article25. Kol S, Lightman A, Hillensjo T, Devroey P, Fauser B, Tarlatzis B, Mannaerts B, Itskovitz-Eldor J. High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze-thaw cycles. Hum Reprod. 1999. 14:2242–2244.
Article26. Lee JR, Choi YS, Jee BC, Ku SY, Suh CS, Kim KC, Lee WD, Kim SH. Cryopreserved blastocyst transfer: impact of gonadotropin-releasing hormone agonist versus antagonist in the previous oocyte retrieval cycles. Fertil Steril. 2007. 88:1344–1349.
Article27. Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, Murphy C, Adams S, Riesewijk A, Mannaerts B, Pellicer A. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005. 20:3318–3327.
Article28. Saadat P, Boostanfar R, Slater CC, Tourgeman DE, Stanczyk FZ, Paulson RJ. Accelerated endometrial maturation in the luteal phase of cycles utilizing controlled ovarian hyperstimulation: impact of gonadotropin-releasing hormone agonists versus antagonists. Fertil Steril. 2004. 82:167–171.
Article29. Chen HJ, Lin YH, Hsieh BC, Seow KM, Hwang JL, Tzeng CR. Is a lower dose of cetrorelix acetate effective for prevention of LH surge during controlled ovarian hyperstimulation? J Assist Reprod Genet. 2006. 23:289–292.
Article30. Messinis IE, Loutradis D, Domali E, Kotsovassilis CP, Papastergiopoulou L, Kallitsaris A, Drakakis P, Dafopoulos K, Milingos S. Alternate day and daily administration of GnRH antagonist may prevent premature luteinization to a similar extent during FSH treatment. Hum Reprod. 2005. 20:3192–3197.
Article31. Huirne JA, van Loenen AC, Schats R, McDonnell J, Hompes PG, Schoemaker J, Homburg R, Lambalk CB. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod. 2005. 20:359–367.
Article32. Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007. 13:343–355.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cessation of gonadotropin-releasing hormone antagonist on triggering day in flexible multiple-dose protocol: A randomized controlled study
- A Study of Clinical Efficacy of GnRH Antagonist (Cetrorelix) Single and Multiple Dose Protocol for Controlled Ovarian Hyperstimulation
- An Update of Gonadotropin-Releasing Hormone Antagonists in the Treatment of Advanced Prostate Cancer
- Response of Human Chorionic Gonadotropin to 6-D-Trp-Gonadotropin-Releasing Hormone and Gonadotropin-Releasing Hormone Stimulation in the Culture Media of Normal Human Placenta of Different Gestational Ages
- A study of low dose purified follicle-stimulating hormone supplemented with gonadotropin releasing hormone agonist in women with polycystic ovarian disease