J Korean Med Sci.
2005 Jun;20(3):461-467. 10.3346/jkms.2005.20.3.461.
Clinical Effectiveness of Urinary Human Chorionic Gonadotropin Related Protein (hCGRP) Quantification for Diagnosis of Ectopic Pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University School of Medicine, Seoul, Korea. jklee38@kumc.or.kr
- 2Samsung Cheil Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, College of Medicine Pochon CHA University, Seoul, Korea.
- 4Humasis Research Center, Gunpo, Korea.
- 5Department of Obstetrics and Gynecology, Thomas Jefferson University, Philadelphia, PA, USA.
- KMID: 1778507
- DOI: http://doi.org/10.3346/jkms.2005.20.3.461
Abstract
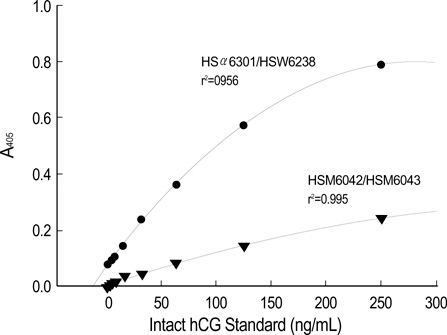
- We detected pregnancy related new molecule, human chorionic gonadotropin related protein (hCGRP) in the urine of a pregnant women by using a monoclonal antibody against the human chorionic gonadotropin (hCG). This study examined the effectiveness of urinary hCGRP quantification in diagnosing ectopic pregnancy. This study included 40 normal pregnant women and 25 patients with ectopic pregnancy. Patients' serum and urinary intact whole hCG (i-hCG) and hCGRP concentrations were measured using sandwich ELISA and the ratio of hCGRP to i-hCG was calculated. Statistical analysis was performed using statistical package for social sciences (SPSS) 10.0. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the cut-off value to discriminate ectopic pregnancies from normal intrauterine pregnancies. Urinary hCGRP and hCGRP/i-hCG ratio in ectopic pregnancy group (14 +/- 6.6 ng/mL, 4.6 +/- 1.9%, respectively) were significantly lower than those of normal pregnancy group (149 +/- 10.2 ng/mL, 29.7 +/- 1.9%, respectively; p<0.001). Based on ROC curve analysis, a cut-off point of urinary hCGRP/i-hCG ratio <16.2% discriminated between ectopic pregnancy and normal pregnancy with a sensitivity, specificity, positive predictive value and negative predictive value of 92.0%, 90.0%, 32.6%, and 99.5%, respectively. Urinary hCGRP/i-hCG ratio measurement may be effective in diagnosing ectopic pregnancy.
Keyword
MeSH Terms
Figure
Reference
-
1. Centers for Disease Control and Prevention. Ectopic pregnancy-United States, 1988-1989. MMWR. 1992. 41:591–594.2. Centers for Disease Control and Prevention. Ectopic pregnancy-United States, 1990-1992. MMWR. 1995. 44:46–48.3. Storeide O, Veholmen M, Eide M, Bergsjo P, Sandvei R. The incidence of ectopic pregnancy in Hordaland County, Norway 1976-1993. Acta Obstet Gynecol Scand. 1997. 76:345–349.
Article4. Simms I, Rogers PA, Nicoll A. The influence of demographic change and cumulative risk of pelvic inflammatory disease on the incidence of ectopic pregnancy. Epidemiol Infect. 1997. 119:49–52.
Article5. Centers for Disease Control and Prevention. Ectopic pregnancy-United States, 1970-1989. MMWR. 1993. 42:73–85.6. Cheon MJ, Kwon YI, Lew YO, Lee BH, Lee HJ, Kim CJ, Kwon DJ, Lee JW, Park TC. A clinical study of ectopic pregnancy during recent 5 years. Korean J Obst Gynecol. 2001. 44:283–289.7. Tak BM, Kim KM, Ryu HK, Yang KS, Chung HS. A clinical study on ectopic pregnancy. Korean J Obst Gynecol. 1998. 41:819–828.
Article8. McCord ML, Muram D, Buster JE, Arheart KL, Stovall TG, Carson SA. Single serum progesterone as a screen for ectopic pregnancy: exchanging specificity and sensitivity to obtain optimal test performance. Fertil Steril. 1996. 66:513–516.
Article9. Ledger WL, Sweeting VM, Chatterjee S. Rapid diagnosis of early ectopic pregnancy in an emergency gynaecology service--are measurements of progesterone, intact and free beta human chorionic gonadotrophin helpful? Human Reprod. 1994. 9:157–160.10. Cole LA, Kardana A, Park SY, Braunstein GD. The deactivation of hCG by nicking and dissociation. J Clin Endocrinol Metab. 1993. 73:704–710.
Article11. Cole LA, Isozaki T, Jones EE. Urine β-core fragment, a potential screening test for ectopic pregnancy and spontaneous abortion. Fetal Diagn Ther. 1997. 12:336–339.
Article12. Stovall T, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med. 1990. 19:1098–1103.
Article13. Barnhart KT, Simhan H, Kamelle SA. Diagnostic accuracy of ultrasound above and below the β-hCG discriminatory zone. Obstet Gynecol. 1999. 94:583–587.14. Garcia CR, Barnhart KT. Diagnosing ectopic pregnancy: Decision analysis comparing six strategies. Obstet Gynecol. 2001. 97:464–470.15. Birken S, Kovalevskaya G, O'Connor J. Immunochemical measurement of early pregnancy isoforms of HCG: potential applications to fertility research, prenatal diagnosis, and cancer. Arch Med Res. 2001. 32:635–643.16. Fein HG, Rosen RW, Weintraub BD. Increased glycosylation of serum human chorionic gonadotropin and subunits from eutopic and ectopic sources; comparison with placental and urinary forms. J Clin Endocrinol Metab. 1980. 50:1111–1120.
Article17. Wide L, Hobson B. Some qualitative differences of hCG in serum from early and late pregnancies and trophoblastic diseases. Acta Endocrinol (Copenh). 1987. 116:465–472.
Article18. Wide L, Lee JY, Rasmussen C. A change in the isoforms of human chorionic gonadotropin occurs around the 13th weeks of gestation. J Clin Endocrinol Metab. 1994. 78:1419–1423.19. Norman RJ, Buck RH, Kemp MA, Joubert SM. Impaired corpus luteum function in ectopic pregnancy cannot be explained by altered human chorionic gonadotropin. J Clin Endocrinol Metab. 1988. 66:1166–1170.
Article20. Johnson MR, Riddle AF, Irvine R, Sharma V, Collins WP, Nicolaides KH, Grudzinskas JG. Corpus luteum failure in ectopic pregnancy. Hum Reprod. 1993. 8:1491–1495.21. Lower AM, Yovich JL, Hancock C, Grudzinskas JG. Is luteal function maintained by factors other than chorionic gonadotropin in early pregnancy? Hum Reprod. 1993. 8:645–648.22. Kratzer PG, Taylor RN. Corpus luteum function in early pregnancies is primarily determined by the rate of change of human chorionic gonadotropin levels. Am J Obstet Gynecol. 1990. 163:1497–1502.
Article23. Johnson MR, Riddle AF, Sharma V, Collins WP, Nicolaides KH, Grudzinskas JG. Placental and ovarian hormones in an embryonic pregnancy. Hum Reprod. 1993. 8:112–115.24. Cole LA. Immunoassay of human chorionic gonadotropin. Its free subunits and metabolites. Clin Chem. 1997. 43:2233–2243.
Article25. Borrelli P, Butler S, Docherty S, Staite E, Borrelli A, Iles R. Human chorionic gonadotropin isoforms in the diagnosis of ectopic pregnancy. Clin Chem. 2003. 49:2045–2049.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endovaginal sonographic findings correlation with human chorionic gonadotropin levels in the ectopic pregnancies
- Primary Ovarian Pregnancy: A Case Report
- Conservative Treatment of Ectopic Pregnancy with Methotrexate
- Patterns of the decline in serum beta-human chorionic gonadotropin level in patients with tubal pregnancy following surgery by pelviscopy and by laparotomy
- Persistent low-level elevation of serum human chorionic gonadotropin after termination of pregnancy: a rare case of peritoneal trophoblastic implant