Korean J Radiol.
2000 Mar;1(1):25-32. 10.3348/kjr.2000.1.1.25.
Usefulness of Single Voxel Pro ton MR Spectroscopy in the Evaluation of Hippocampal Sclerosis
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. changkh@radcom.snu.ac.kr
- KMID: 1777292
- DOI: http://doi.org/10.3348/kjr.2000.1.1.25
Abstract
OBJECTIVE
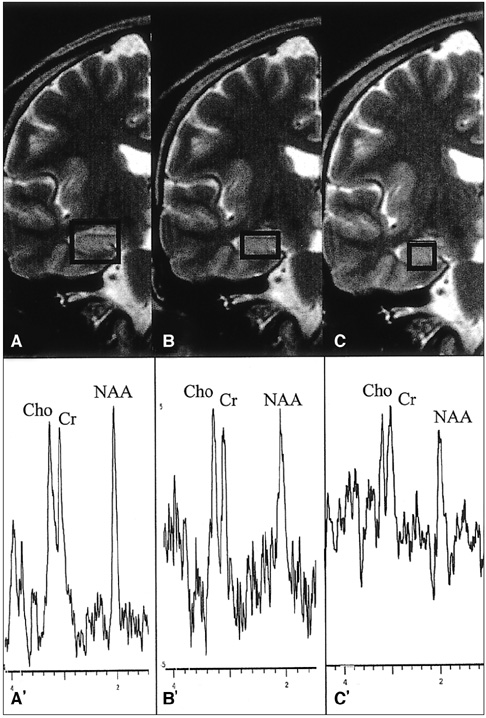
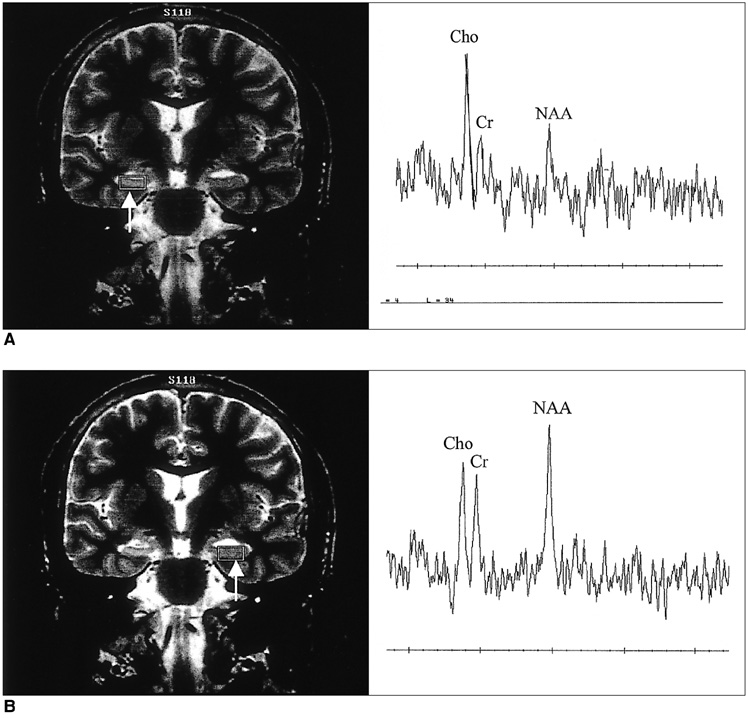
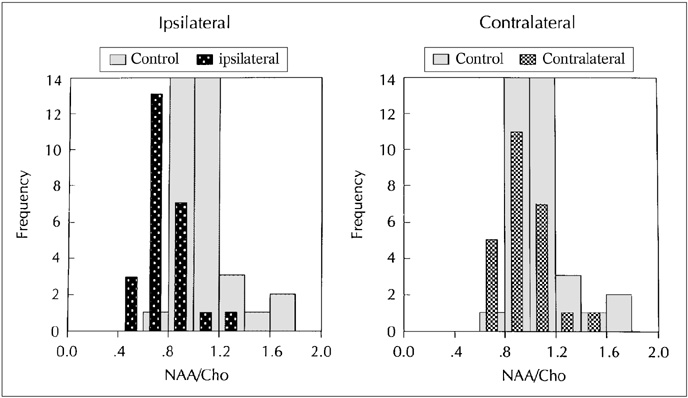
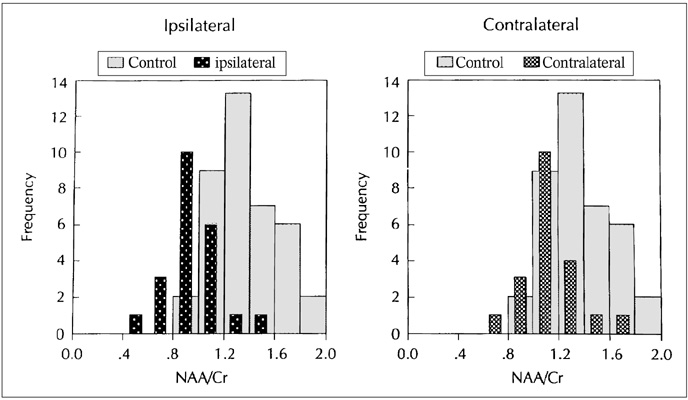
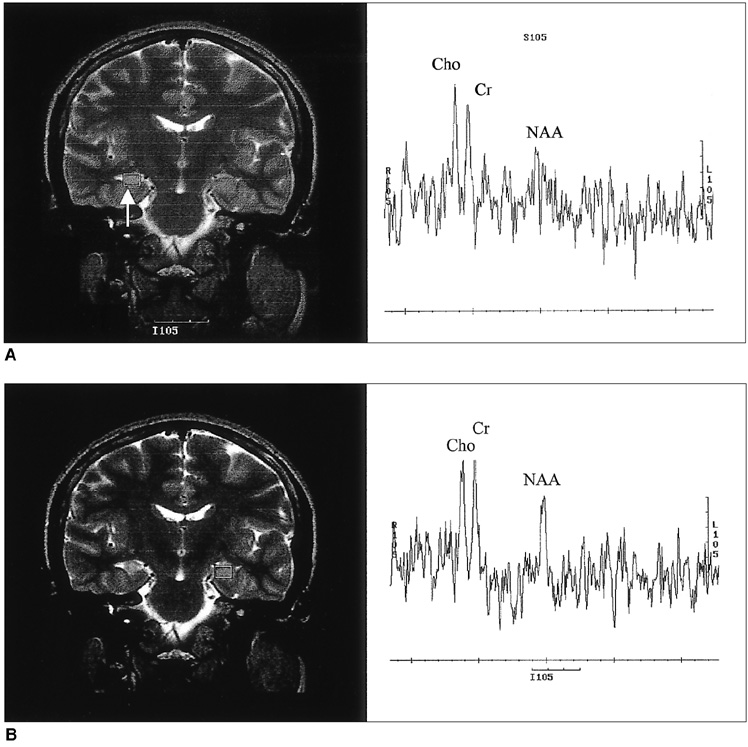
The purpose of our study was to determine the ability of H-1 MR spectroscopy (MRS) to lateralize the lesion in patients with hippocampal sclerosis. MATERIALS AND METHODS: Twenty healthy volunteers and 25 patients with intractable temporal lobe epilepsy whose MR imaging diagnosis was unilateral hippocampal sclerosis were included. This diagnosis was based on the presence of unilateral atrophy and/or high T2 signal intensity of the hippocampus. Single-voxel H-1 MRS was carried out on a 1.5-T unit using PRESS sequence (TE, 136 msec). Spectra were obtained from hippocampal areas bilaterally with volumes of interest (VOIs) of 6.0 cm 3and 2.25 cm 3 in healthy volunteers, and of either 6.0 c m 3 (n = 14) or 2.25 cm 3 (n = 11) in patients. Metabolite ratios of NAA/Cho and NAA/Cr were calculated from relative peak height measurements. The capability of MRS to lateralize the lesion and to detect bilateral abnormalities was compared with MR imaging diagnosis as a standard of reference. RESULTS: In healthy volunteers, NAA/Cho and NAA/Cr ratios were greater than 0.8 and 1.0, respectively. In patients, the mean values of these ratios were significantly lower on the lesion side than on the contralateral side, and lower than those of healthy volunteers (p <.05). The overall correct lateralization rate of MRS was 72% (18/25); this rate was lower with a VOI of 6.0 cm 3 than of 2.25 cm 3 (64% versus 82%, p <.05). Bilateral abnormalities on MRS were found in 24% (6/25) of cases. CONCLUSION: Although its rate of correct lateralization is low, single-voxel H-1 MRS is a useful and promising diagnostic tool in the evaluation of hippocampal sclerosis, particularly for the detection of bilateral abnormalities. To improve the diagnostic accuracy of H-1 MRS, further investigation, including the use of a smaller VOI and measurement of the absolute amount of metabolites, are needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Babb TI, Brown WJ. Engel J, editor. Pathological findings in epilepsy. Surgical treatment of the epilepsies. 1987. New York: Raven Press;511–540.2. Bruton CJ. The neuropathology of temporal lobe epilepsy. 1988. Oxford: Oxford Univ. Press.3. Jack CR, Sharbrough FW, Cascino GD, Hirschorn KA, O'Brien PC, Marsh WR. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992. 31:138–146.4. Jack CR. Epilepsy: surgery and imaging. Radiology. 1993. 189:635–646.5. Bronen RA. Epilepsy: the role of MR imaging. AJR. 1992. 159:1165–1174.6. Jayakar P, Duchowny M, Resnick TJ, Alvarez LA. Localization of seizure foci: pitfalls and caveats. J Clin Neurophysiol. 1991. 8:414–431.7. Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994. 44:1411–1417.8. Achten E, Santens P, Boon P, et al. Single-voxel proton MR spectroscopy and positron emission tomography for lateralization of refractory temporal lobe epilepsy. AJNR. 1998. 19:1–8.9. Helveston W, Gilmore R, Roper S, et al. Intractable temporal lobe epilepsy: comparison of positron emission tomography with qualitative and quantitative MR. AJNR. 1996. 17:1515–1521.10. Song IC, Chang KH, Min KH, et al. H-1 MR spectroscopic patterns of normal adult brain. J Korean Radiol Soc. 1996. 35:435–440.11. Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, Bryan RN. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993. 6:89–94.12. Moonen CT, von Kienlin M, Gillen J, et al. Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy. NMR Biomed. 1989. 2:201–208.13. Gadian DG, Connelly A, Duncan JS, et al. 1H magnetic resonance spectroscopy in the investigation of intractable epilepsy. Acta Neurol Scand Suppl. 1994. 152:116–121.14. Cendes F, Andermann F, Preul MC, et al. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol. 1994. 35:211–216.15. Garcia PA, Laxer KD, Ng T. Application of spectroscopic imaging in epilepsy. Magn Reson Imaging. 1995. 13:1181–1185.16. Cross JH, Connelly A, Jackson GD, et al. Proton magnetic resonance spectroscopy in children with temporal lobe epilepsy. Ann Neurol. 1996. 39:107–113.17. Hugg JW, Laxer KD, Matson GB, et al. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1993. 34:788–794.18. Epstein CM, Boor D, Hoffman JC, et al. Evaluation of 1H magnetic resonance spectroscopic imaging as a diagnostic tool for the lateralization of epileptogenic seizure foci. Br J Radiol. 1996. 69:15–24.19. Matthews PM, Andermann F, Arnold DL. A proton magnetic resonance spectroscopy study of focal epilepsy in humans. Neurology. 1990. 40:985–989.20. Thompson JE, Castillo M, Kwock L, Walters B, Beach R. Usefulness of proton MR spectroscopy in the evaluation of temporal lobe epilepsy. AJR. 1998. 170:771–776.21. Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes. Brain. 1966. 89:499–530.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Localized Single-Voxel Spin-Echo Proton MR Spectroscopy of Normal Hippocampal Area at 1.5 T : Optimal Voxel Volume and Reproducibility of Metabolite Ratios
- Hippocampal and Neocortical Metabolite Ratio in Patients with Complex Partial Seizure: Short TE and Long TE Techniques Using Single Voxel Proton MR Spectroscopy
- The Significance and Limitation of MR Volumetry: Comparison between Normal Adults and the Patients with Epilepsy and Hippocampal Sclerosis
- Investigation of Varied MR Spectra by TE and Metabolite Amount in the Localized Voxel using the MR Cone-shape Phantom
- Clinical Usefulness of T2 Relaxometry in Temporal Lobe Epilepsy