Korean J Radiol.
2005 Dec;6(4):229-234. 10.3348/kjr.2005.6.4.229.
Gadobenate Dimeglumine as an Intrabiliary Contrast Agent: Comparison with Mangafodipir Trisodium with Respect to Non-dilated Biliary Tree Depiction
- Affiliations
-
- 1Department of Diagnostic Radiology, Yonsei University College of Medicine, Korea. kimnex@yumc.yonsei.ac.kr
- 2Brain Korea 21 Project of Medical Science, Yonsei University College of Medicine, Korea.
- 3Institute of Gastroenterology, Yonsei University College of Medicine, Korea.
- KMID: 1777280
- DOI: http://doi.org/10.3348/kjr.2005.6.4.229
Abstract
OBJECTIVE
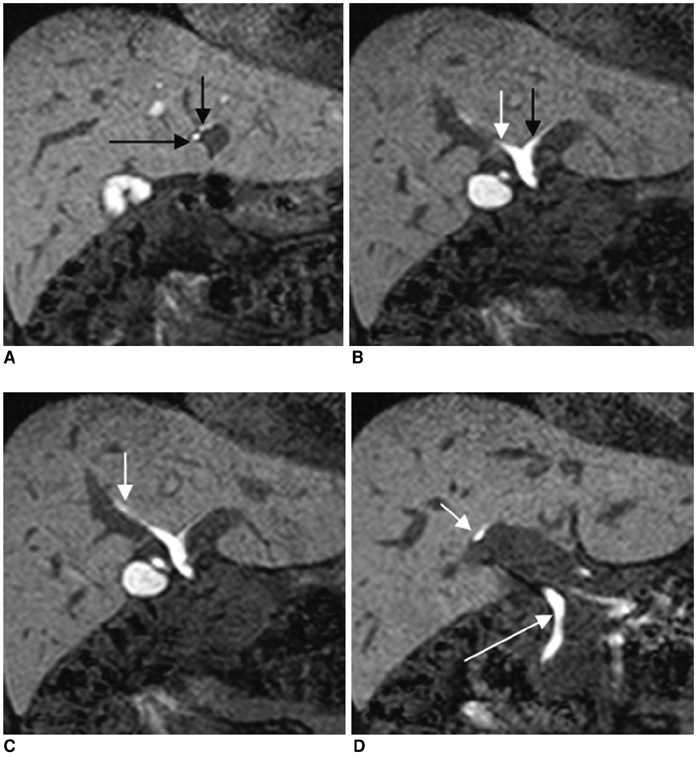
To compare the efficacy of Mangafodipir trisodium (Mn-DPDP) -enhanced MR cholangiogrphy (MRC) and Gadobenate dimeglumine (Gd-BOPTA) -enhanced MRC in visualizing a non-dilated biliary system. MATERIALS AND METHODS: Eighty-eight healthy liver donor candidates underwent contrast-enhanced T1-weighted MRC. Mn-DPDP and Gd-BOPTA was used in 36 and 52 patients, respectively. Two radiologists reviewed the MR images and rated the visualization of the common duct, the right and left hepatic ducts, and the second-order branches using a 4-point scale. The contrast-to-noise ratio (CNR) of the common duct to the liver in the two groups was also compared. RESULTS: Mn-DPDP MRC and Gd-BOPTA MRC both showed similar visualization grades in the common duct (p = .380, Mann-Whitney U test). In the case of the proximal bile ducts, the median visualization grade was significantly higher with Gd-BOPTA MRC than with Mn-DPDP MRC (right hepatic duct: p = 0.016, left hepatic duct: p = 0.014, right secondary order branches: p = 0.006, left secondary order branches, p = 0.003). The common duct-to-liver CNR of the Gd-BOPTA MRC group was significantly higher (38.90+/-24.50) than that of the Mn-DPDP MRC group (24.14+/-17.98) (p = .003, Student's t test). CONCLUSION: Gd-BOPTA, as a biliary contrast agent, is a potential substitute for Mn-DPDP.
Keyword
MeSH Terms
-
Pyridoxal Phosphate/*analogs & derivatives/diagnostic use
Organometallic Compounds/*diagnostic use
Middle Aged
Meglumine/*analogs & derivatives/diagnostic use
Male
*Magnetic Resonance Imaging
Humans
Hepatic Duct, Common/anatomy & histology
Female
Feasibility Studies
Edetic Acid/*analogs & derivatives/diagnostic use
*Contrast Media
Common Bile Duct/anatomy & histology
Bile Ducts/*anatomy & histology
Aged
Adult
Figure
Reference
-
1. Takehara Y. Fast MR imaging for evaluating the pancreaticobiliary system. Eur J Radiol. 1999. 29:211–232.2. Irie H, Honda H, Tajima T, Kuroiwa T, Yoshimitsu K, Makisumi K, et al. Optimal MR cholangiopancreatographic sequence and its clinical application. Radiology. 1998. 206:379–387.3. Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998. 207:21–32.4. Choi JW, Kim TK, Kim KW, Kim AY, Kim PN, Ha HK, et al. Anatomic variation in intrahepatic bile ducts: an analysis of intraoperative cholangiograms in 300 consecutive donors for living donor liver transplantation. Korean J Radiol. 2003. 4:85–90.5. Hintze RE, Adler A, Veltzke W, Abou-Rebyeh H, Hammerstingl R, Vogl T, et al. Clinical significance of magnetic resonance cholangiopancreatography (MRCP) compared to endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy. 1997. 29:182–187.6. Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics. 2001. 21:23–37.7. Mitchell DG, Alam F. Mangafodipir trisodium: effects on T2- and T1-weighted MR cholangiography. J Magn Reson Imaging. 1999. 9:366–368.8. Papanikolaou N, Prassopoulos P, Eracleous E, Maris T, Gogas C, Gourtsoyiannis N. Contrast-enhanced magnetic resonance cholangiography versus heavily T2-weighted magnetic resonance cholangiography. Invest Radiol. 2001. 36:682–686.9. Lee VS, Rofsky NM, Morgan GR, Teperman LW, Krinsky GA, Berman P, et al. Volumetric mangafodipir trisodium-enhanced cholangiography to define intrahepatic biliary anatomy. AJR Am J Roentgenol. 2001. 176:906–908.10. Carlos RC, Hussain HK, Song JH, Francis IR. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid as an intrabiliary contrast agent: preliminary assessment. AJR Am J Roentgenol. 2002. 179:87–92.11. Kapoor V, Peterson MS, Baron RL, Patel S, Eghtesad B, Fung JJ. Intrahepatic biliary anatomy of living adult liver donors: correlation of mangafodipir trisodium-enhanced MR cholangiography and intraoperative cholangiography. AJR Am J Roentgenol. 2002. 179:1281–1286.12. Vitellas KM, El-Dieb A, Vaswani KK, Bennett WF, Fromkes J, Ellison C, et al. Using contrast-enhanced MR cholangiography with IV mangafodipir trisodium (Teslascan) to evaluate bile duct leaks after cholecystectomy: a prospective study of 11 patients. AJR Am J Roentgenol. 2002. 179:409–416.13. Kim KW, Park MS, Yu JS, Chung JP, Ryu YH, Lee SI, et al. Acute cholecystitis at T2-weighted and manganese-enhanced T1-weighted MR cholangiography: preliminary study. Radiology. 2003. 227:580–584.14. Marinelli ER, Neubeck R, Song B, Wagler T, Ranganathan RS, Sukumaran K, et al. Synthesis, characterization, and imaging performance of a new class of macrocyclic hepatobiliary MR contrast agents. Invest Radiol. 2000. 35:8–24.15. Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol. 1998. 33:798–809.16. Lorusso V, Arbughi T, Tirone P, de Haen C. Pharmacokinetics and tissue distribution in animals of gadobenate ion, the magnetic resonance imaging contrast enhancing component of gadobenate dimeglumine 0.5 M solution for injection (MultiHance). J Comput Assist Tomogr. 1999. 23:Suppl 1. S181–S194.17. Spinazzi A, Lorusso V, Pirovano G, Kirchin M. Safety, tolerance, biodistribution, and MR imaging enhancement of the liver with gadobenate dimeglumine: results of clinical pharmacologic and pilot imaging studies in nonpatient and patient volunteers. Acad Radiol. 1999. 6:282–291.18. Elizondo G, Fretz CJ, Stark DD, Rocklage SM, Quay SC, Worah D, et al. Preclinical evaluation of MnDPDP: new paramagnetic hepatobiliary contrast agent for MR imaging. Radiology. 1991. 178:73–78.19. Hamm B, Vogl TJ, Branding G, Schnell B, Taupitz M, Wolf KJ, et al. Focal liver lesions: MR imaging with Mn-DPDP-initial clinical results in 40 patients. Radiology. 1992. 182:167–174.20. Slater GJ, Saini S, Mayo-smith WW, Sharma P, Eisenberg PJ, Hahn PF. Mn-DPDP enhanced MR imaging of the liver: analysis of pulse sequence performance. Clin Radiol. 1996. 51:484–486.21. Wang C, Gordon PB, Hustvedt SO, Grant D, Sterud AT, Martinsen I, et al. MR imaging properties and pharmacokinetics of MnDPDP in healthy volunteers. Acta Radiol. 1997. 38(4 Pt 2):665–676.22. Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004. 14:559–578.23. Caudana R, Morana G, Pirovano GP, Nicoli N, Portuese A, Spinazzi A, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium benzyloxypropionictetra-acetate (BOPTA)-preliminary results of phase II clinical application. Radiology. 1996. 199:513–520.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypointense Hepatic Lesions Depicted on Gadobenate Dimeglumine-Enhanced Three-Hour Delayed Hepatobiliary-Phase MR Imaging: Differentiation between Benignancy and Malignancy
- Usefulness of Gadobenate Dimeglumine - Enhanced Hepatobiliary Phase MR Imaging on Predicting Histological Grade of Hepatocellular Carcinoma
- Two cases of Nephrotic Syndrome Associated with Kimura's Disease
- Gadoxetic Acid (Gd-EOB-DTPA)-Enhanced MRI versus Gadobenate Dimeglumine (Gd-BOPTA)-Enhanced MRI for Preoperatively Detecting Hepatocellular Carcinoma: an Initial Experience
- Embryonal Rhabdomyosarcoma of the Biliary Tree: A Case Report