Yonsei Med J.
2006 Dec;47(6):852-861. 10.3349/ymj.2006.47.6.852.
Effects of Sinusoidal Electromagnetic Field on Structure and Function of Different Kinds of Cell Lines
- Affiliations
-
- 1Department of Medical Engineering, Yonsei University College of Medicine, Seoul, Korea. hwal@yumc.yonsei.ac.kr
- 2National BK21 Project Team of Nanobiomaterials for the Cell-based Implants, Seoul, Korea.
- KMID: 1777176
- DOI: http://doi.org/10.3349/ymj.2006.47.6.852
Abstract
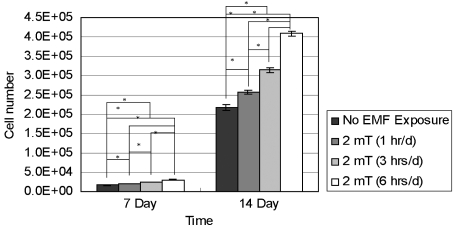
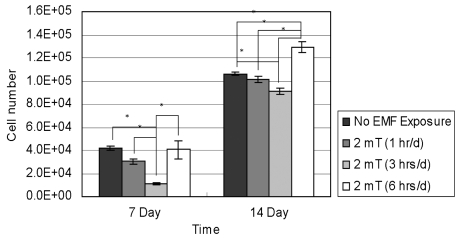
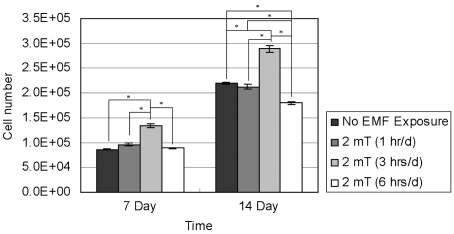
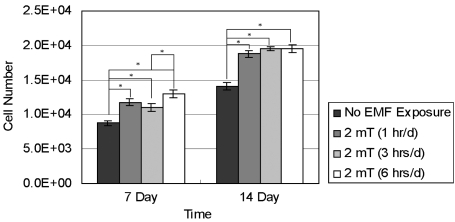
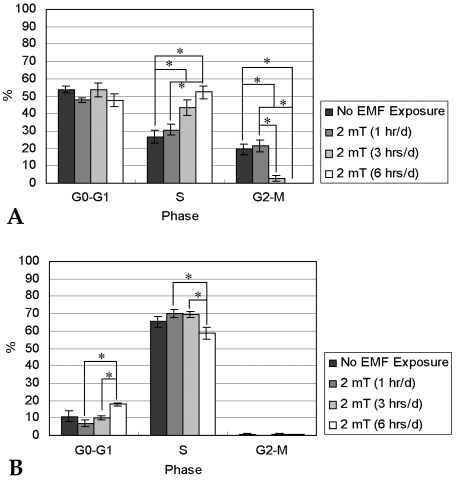
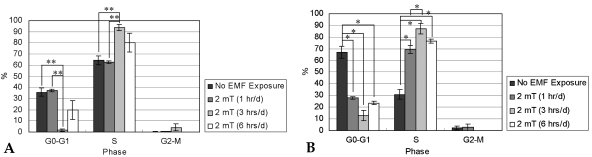
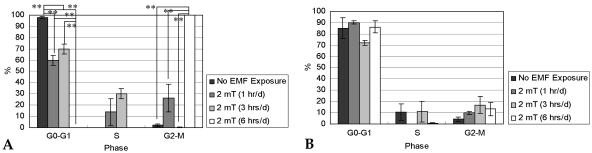
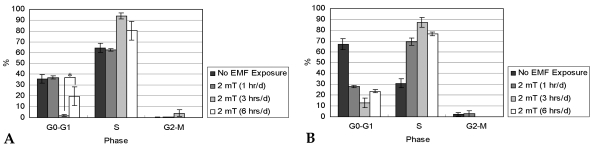
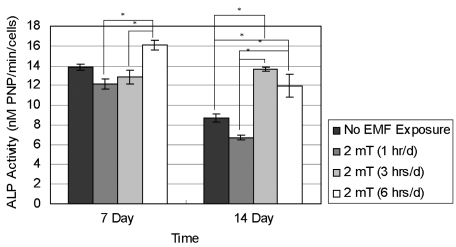
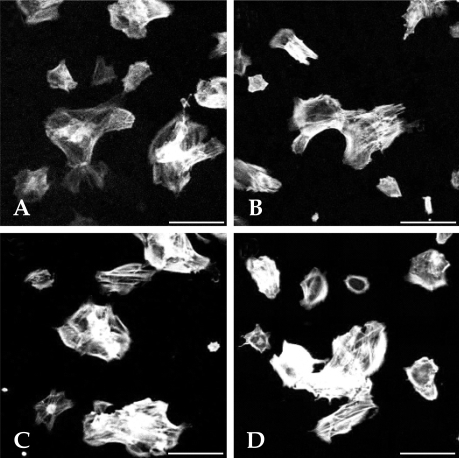
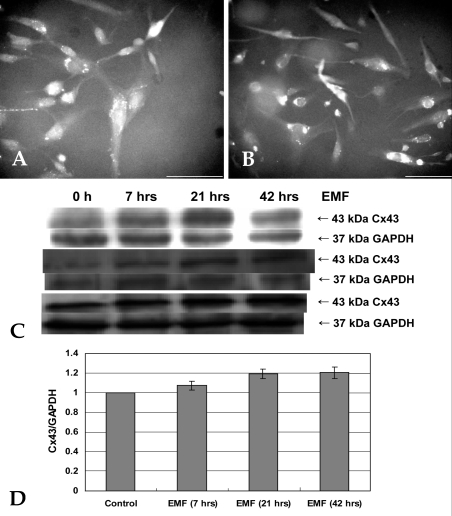
- This study investigated that whether a 2 mT, 60 Hz, sinusoidal electromagnetic field (EMF) alters the structure and function of cells. This research compared the effects of EMF on four kinds of cell lines: hFOB 1.19 (fetal osteoblast), T/G HA-VSMC (aortic vascular smooth muscle cell), RPMI 7666 (B lymphoblast), and HCN-2 (cortical neuronal cell). Over 14 days, cells were exposed to EMF for 1, 3, or 6 hours per day (hrs/d). The results pointed to a cell type-specific reaction to EMF exposure. In addition, the cellular responses were dependent on duration of EMF exposure. In the present study, cell proliferation was the trait most sensitive to EMF. EMF treatment promoted growth of hFOB 1.19 and HCN-2 compared with control cells at 7 and 14 days of incubation. When the exposure time was 3 hrs/d, EMF enhanced the proliferation of RPMI 7666 but inhibited that of T/G HA- VSMC. On the other hand, the effects of EMF on cell cycle distribution, cell differentiation, and actin distribution were unclear. Furthermore, we hardly found any correlation between EMF exposure and gap junctional intercellular communication in hFOB 1.19. This study revealed that EMF might serve as a potential tool for manipulating cell proliferation.
Keyword
MeSH Terms
Figure
Reference
-
1. Bellavite P, Signorini A. Schulte J, Endle PC, editors. Biological effects of electromagnetic fields. Fundamental research in ultra-high dilution and homoeopathy. 1998. Dordrecht: Kluwer Academic Publisher;p. 127–142.
Article2. Goodman R, Blank M. Insights into electromagnetic interaction mechanisms. J Cell Physiol. 2002; 192:16–22. PMID: 12115732.
Article3. Manni V, Lisi A, Rieti S, Serafino A, Ledda M, Giuliani L, et al. Low electromagnetic field (50 Hz) induces differentiation on primary human oral keratinocytes (HOK). Bioelectromagnetics. 2004; 25:118–126. PMID: 14735562.
Article4. Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res. 2002; 20:40–50. PMID: 11853089.5. Schimmelpfeng J, Dertinger H. Action of a 50 Hz magnetic field on proliferation of cells in culture. Bioelectromagnetics. 1997; 18:177–183. PMID: 9084869.
Article6. Chang WH, Chen LT, Sun JS, Lin FH. Effect of pulseburst electromagnetic field stimulation on osteoblast cell activities. Bioelectromagnetics. 2004; 25:457–465. PMID: 15300732.
Article7. Sul AR, Park SN, Suh H. Effects of electromagnetic fields on structure and function of rat glioma cell line. Res J Microbiol. 2006; 1:124–135.8. Wei M, Guizzetti M, Yost M, Costa LG. Exposure to 60-Hz magnetic fields and proliferation of human astrocytoma cells in vitro. Toxicol Appl Pharmacol. 2000; 162:166–176. PMID: 10652245.9. Aldinucci C, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, et al. The effect of pulsed electromagnetic fields on the physiologic behaviour of a human astrocytoma cell line. Biochim Biophys Acta. 2000; 1499:101–108. PMID: 11118642.
Article10. Pessina GP, Aldinucci C, Palmi M, Sgaragli G, Benocci A, Meini A, et al. Pulsed electromagnetic fields affect the intracellular calcium concentrations in human astrocytoma cells. Bioelectromagnetics. 2001; 22:503–510. PMID: 11568936.
Article11. Lee JH, McLeod KJ. Morphologic responses of osteoblast-like cells in monolayer culture to ELF electromagnetic fields. Bioelectromagnetics. 2000; 21:129–136. PMID: 10653624.
Article12. Manni V, Lisi A, Pozzi D, Rieti S, Serafino A, Giuliani L, et al. Effects of extremely low frequency (50 Hz) magnetic field on morphological and biochemical properties of human keratinocytes. Bioelectromagnetics. 2002; 23:298–305. PMID: 11948610.
Article13. Ivancsits S, Pilger A, Diem E, Jahn O, Rudiger HW. Cell type-specific genotoxic effects of intermittent extremely low-frequency electromagnetic fields. Mutat Res. 2005; 583:184–188. PMID: 15899587.
Article14. Berg H. Problems of weak electromagnetic field effects in cell biology. Bioelectrochem Bioenerg. 1999; 48:355–360. PMID: 10379554.
Article15. Hannay G, Leavesley D, Pearcy M. Timing of pulsed electromagnetic field stimulation does not affect the promotion of bone cell development. Bioelectromagnetics. 2005; 26:670–676. PMID: 16189825.
Article16. De Mattei M, Caruso A, Traina GC, Pezzetti F, Baroni T, Sollazzo V. Correlation between pulsed electromagnetic fields exposure time and cell proliferation increase in human osteosarcoma cell lines and human normal osteoblast cells in vitro. Bioelectromagnetics. 1999; 20:177–182. PMID: 10194560.17. Schiller PC, D'Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001; 28:362–369. PMID: 11336916.18. Zeng QL, Chiang H, Hu GL, Mao GG, Fu YT, Lu DQ. ELF magnetic fields induce internalization of gap junction protein connexin 43 in Chinese hamster lung cells. Bioelectromagnetics. 2003; 24:134–138. PMID: 12524680.
Article19. Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon BJ, Sylvia VL, et al. Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res. 2003; 21:326–334. PMID: 12568966.
Article20. Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004; 419:30–37. PMID: 15021128.
Article21. Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984; 67:379–388. PMID: 6200537.
Article22. Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975; 66:188–193. PMID: 49354.
Article23. Gramsch B, Gabriel HD, Wiemann M, Grummer R, Winterhager E, Bingmann D, et al. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res. 2001; 264:397–407. PMID: 11262196.
Article24. Santoro N, Lisi A, Pozzi D, Pasquali E, Serafino A, Grimaldi S. Effect of extremely low frequency (ELF) magnetic field exposure on morphological and biophysical properties of human lymphoid cell line (Raji). Biochim Biophys Acta. 1997; 1357:281–290. PMID: 9268052.
Article25. Lisi A, Pozzi D, Pasquali E, Rieti S, Girasole M, Cricenti A, et al. Three dimensional (3D) analysis of the morphological changes induced by 50 Hz magnetic field exposure on human lymphoblastoid cells (Raji). Bioelectromagnetics. 2000; 21:46–51. PMID: 10615091.
Article26. Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA. 1979; 76:4498–4502. PMID: 291981.
Article27. Roymans D, Grobben B, Claes P, Slegers H. Protein tyrosine kinase-dependent regulation of adenylate cyclase and phosphatidylinositol 3-kinase activates the expression of glial fibrillary acidic protein upon induction of differentiation in rat C6 glioma. Cell Biol Int. 2001; 25:467–474. PMID: 11401334.
Article28. Mangiacasale R, Tritarelli A, Sciamanna I, Cannone M, Lavia P, Barberis MC, et al. Normal and cancer-prone human cells respond differently to extremely low frequency magnetic fields. FEBS Lett. 2001; 487:397–403. PMID: 11163365.
Article29. Lin Y, Wong K, Calame K. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science. 1997; 276:596–599. PMID: 9110979.30. Tokalov SV, Gutzeit HO. Weak electromagnetic fields (50 Hz) elicit a stress response in human cells. Environ Res. 2004; 94:145–151. PMID: 14757377.31. Cridland NA, Haylock RG, Saunders RD. 50 Hz magnetic field exposure alters onset of S-phase in normal human fibroblasts. Bioelectromagnetics. 1999; 20:446–452. PMID: 10495310.
Article32. Chen G, Upham BL, Sun W, Chang CC, Rothwell EJ, Chen KM, et al. Effect of electromagnetic field exposure on chemically induced differentiation of friend erythroleukemia cells. Environ Health Perspect. 2000; 108:967–972. PMID: 11049817.
Article33. Lohmann CH, Schwartz Z, Liu Y, Guerkov H, Dean DD, Simon B, et al. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J Orthop Res. 2000; 18:637–646. PMID: 11052501.
Article34. Hartig M, Joos U, Wiesmann HP. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur Biophys J. 2000; 29:499–506. PMID: 11156291.35. Grace KL, Revell WJ, Brookes M. The effects of pulsed electromagnetism on fresh fracture healing: osteochondral repair in the rat femoral groove. Orthopedics. 1998; 21:297–302. PMID: 9547814.
Article36. Norton LA. Effects of a pulsed electromagnetic field on a mixed chondroblastic tissue culture. Clin Orthop Relat Res. 1982; 167:280–290. PMID: 7094473.
Article37. Diniz P, Shomura K, Soejima K, Ito G. Effects of pulsed electromagnetic field (PEMF) stimulation on bone tissue like formation are dependent on the maturation stages of the osteoblasts. Bioelectromagnetics. 2002; 23:398–405. PMID: 12111759.
Article38. Aaron RK, Ciombor DM. Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res. 1996; 14:582–589. PMID: 8764867.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of static magnetic field and pulsed electromagnetic field on alkaline phosphatase and dna synthetic activity of ME3t3-E1 cells
- Measurement and Modeling of Personal Exposure to the Electric and Magnetic Fields in the Vicinity of High Voltage Power Lines
- Effect of electromagnetic field exposure on the reproductive system
- The Role of Low-Frequency Electromagnetic Fields on Mesenchymal Stem Cells Differentiation: A Systematic Review
- The Effects of Electromagnetic Field on Distraction Osteogenesis