Yonsei Med J.
2009 Dec;50(6):784-788. 10.3349/ymj.2009.50.6.784.
Withdrawal of Immunosuppression in Pediatric Liver Transplant Recipients in Korea
- Affiliations
-
- 1Department of Pediatrics, Kangnam Sacred Heart Hospital, Hallym University School of Medicine, Seoul, Korea.
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. i101016@skku.edu
- KMID: 1777091
- DOI: http://doi.org/10.3349/ymj.2009.50.6.784
Abstract
- PURPOSE
We identified pediatric liver transplant recipients with successful withdrawal of immunosuppression who developed tolerance in Korea.
MATERIALS AND METHODS
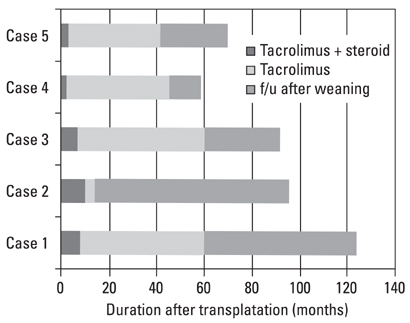
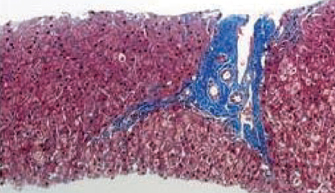
Among 105 pediatric patients who received liver transplantation and were treated with tacrolimus-based immunosuppressive regimens, we selected five (4.8%) patients who had very low tacrolimus trough levels. Four of them were noncompliant with their medication and one was weaned off of immunosuppression due to life threatening posttransplant lymphoproliferative disorder. We reviewed the medical records with regard to the relationship of the donor-recipients, patient characteristics and prognosis, including liver histology, and compared our data with previous reports. RESULTS: Four patients received the liver transplantation from a parent donor and one patient from a cadaver donor. A trial of withdrawal of the immunosuppressant was started a median of 45 months after transplantation (range, 14 months to 60 months), and the period of follow up after weaning from the immunosuppressant was a median of 32 months (range, 14 months to 82 months). None of the five patients had rejection episodes after withdrawal of the immunosuppression; they maintained normal graft function for longer than 3 years (median, 38 months; range, 4 to 53 months). The histological findings of two grafts 64 and 32 months after weaning-off of the medication showed no evidence of chronic rejection.
CONCLUSION
The favorable markers for successful withdrawal of immunosuppression were 1) long-term (> 3 years) stable graft function, 2) no rejection for longer than 1 year after withdrawal of immunosuppression, 3) non-immune mediated liver diseases, and 4) pediatric patients.
MeSH Terms
Figure
Reference
-
1. Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006. 6:1774–1780.
Article2. Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998. 27:926–933.
Article3. Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001. 72:449–454.
Article4. Thomson AW, Mazariegos GV, Reyes J, Donnenberg VS, Donnenberg AD, Bentlejewski C, et al. Monitoring the patient off immunosuppression. Conceptual framework for a proposed tolerance assay study in liver transplant recipients. Transplantation. 2001. 72:8 Suppl. S13–S22.5. Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992. 339:1579–1582.
Article6. Pons JA, Yélamos J, Ramírez P, Oliver-Bonet M, Sánchez A, Rodríquez-Gago M, et al. Endothelial cell chimerism does not influence allograft tolerance in liver transplant patients after withdrawal of immunosuppression. Transplantation. 2003. 75:1045–1047.
Article7. Tryphonopoulos P, Tzakis AG, Weppler D, Garcia-Morales R, Kato T, Madariaga JR, et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant. 2005. 5:608–613.
Article8. Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003. 3:689–696.
Article9. Hurwitz M, Desai DM, Cox KL, Berquist WE, Esquivel CO, Millan MT. Complete immunosuppressive withdrawal as a uniform approach to post-transplant lymphoproliferative disease in pediatric liver transplantation. Pediatr Transplant. 2004. 8:267–272.
Article10. Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: is it worth the risk? Transplantation. 2005. 79:1157–1159.
Article11. Massarollo PC, Mies S, Abdala E, Leitão RM, Raia S. Immunosuppression withdrawal for treatment of severe infections in liver transplantation. Transplant Proc. 1998. 30:1472–1474.
Article12. Mazariegos GV, Reyes J, Marino I, Flynn B, Fung JJ, Starzl TE. Risks and benefits of weaning immunosuppression in liver transplant recipients: long-term follow-up. Transplant Proc. 1997. 29:1174–1177.
Article13. Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997. 63:243–249.
Article14. Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006. 44:702–709.
Article15. Sebagh M, Rifai K, Féray C, Yilmaz F, Falissard B, Roche B, et al. All liver recipients benefit from the protocol 10-year liver biopsies. Hepatology. 2003. 37:1293–1301.
Article16. Koshiba T, Li Y, Takemura M, Wu Y, Sakaquchi S, Minato N, et al. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007. 17:94–97.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric Liver Transplantation
- Operation tolerance after liver transplantation
- Immunosuppression for liver transplant recipients during the COVID-19 pandemic
- Safety and metabolic advantages of steroid withdrawal after 6-months post-transplant in de novo kidney transplantation: 1-year prospective cohort study
- Anesthetic Management in Pediatric Liver Transplantation