J Bacteriol Virol.
2014 Dec;44(4):336-341. 10.4167/jbv.2014.44.4.336.
Seroepidemiological Survey of Aujeszky's Disease Virus in Wild Boar (Sus scrofa) and Raccoon Dogs (Nyctereutes procyonoides koreensis) in Korea
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Anyang, Korea. yangdk@korea.kr
- 2College of Veterinary Medicine, Kangwon National University, Korea.
- 3Wild Life Center, Gyeonggi-do Veterinary Service Laboratory Korea, Korea.
- KMID: 1737322
- DOI: http://doi.org/10.4167/jbv.2014.44.4.336
Abstract
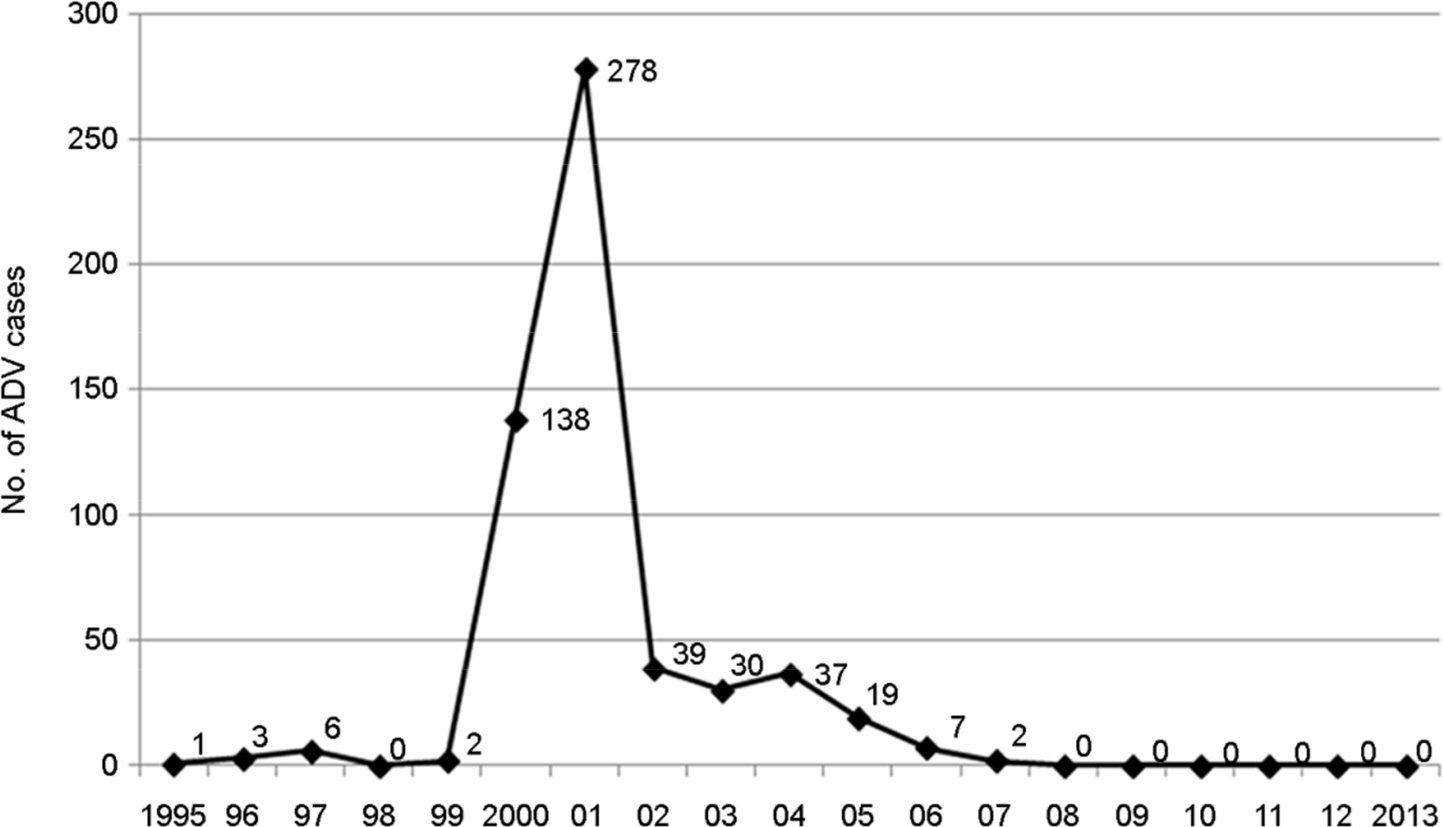
- Aujeszky's disease caused by Aujeszky's disease virus (ADV) is one of the most important diseases in the pig industry. In this study, we conducted a seroepidemiological survey of ADV in wild boars and raccoon dogs in South Korea. In total, 217 wild boar sera collected between March and August 2013, and 96 raccoon dogs between 2011 and 2012 were screened for the presence of antibodies against ADV. The sero-positive rates in wild boars and raccoon dogs tested for ADV were found to be 3.55% (8/225) and 0% (0/96), respectively. The presence of virus neutralization antibody titer against ADV means that small number of wild boars was infected with ADV and AD may be circulated continuously in Korean wild boar populations, and that wild boars may act as a potential reservoir of ADV. Therefore, to achieve the declaration of AD free, effective preventive measures to block transmission of AD should be taken to the wild boars.
Figure
Cited by 1 articles
-
An oral Aujeszky's disease vaccine (YS-400) induces neutralizing antibody in pigs
Dong-Kun Yang, Ha-Hyun Kim, Sung-Suk Choi, Bang-Hun Hyun, Jae-Young Song
Clin Exp Vaccine Res. 2016;5(2):132-137. doi: 10.7774/cevr.2016.5.2.132.
Reference
-
1). Mettenleiter TC. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis-state of the art, June 1999. Vet Res. 2000; 31:99–115.2). Ferrari M, Gualandi GL, Corradi A, Monaci C, Romanelli MG, Tosi G, et al. Experimental infection of pigs with a thymidine kinase negative strain of pseudorabies virus. Comp Immunol Microbiol Infect Dis. 1998; 21:291–303.
Article3). Müller T, Bätza HJ, Schlüter H, Conraths FJ, Mettenleiter TC. Eradication of Aujeszky's disease in Germany. J Vet Med B Infect Dis Vet Public Health. 2003; 50:207–13.4). Song JY, Lee JB, Hyun BH, Park JH, Kim BH, Kweon CH, et al. Glycoprotein g III of Ajesky's disease virus expressed in insect cells by a baculovirus. J Korean Soc Virol. 1992; 22:119–28.5). Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, et al. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011; 156:1691–705.
Article6). Paes Rde C, Fonseca AA Jr, Monteiro LA, Jardim GC, Piovezan U, Herrera HM, et al. Serological and molecular investigation of the prevalence of Aujeszky's disease in feral swine (Sus scrofa) in the subregions of the Pantanal wetland, Brazil. Vet Microbiol. 2013; 165:448–54.7). Boadella M, Gortázar C, Vicente J, Ruiz-Fons F. Wild boar: an increasing concern for Aujeszky's disease control in pigs? BMC Vet Res. 2012; 17(8):7.
Article8). Maresch C, Lange E, Teifke JP, Fuchs W, Klupp B, Müller T, et al. Oral immunization of wild boar and domestic pigs with attenuated live vaccine protects against Pseudorabies virus infection. Vet Microbiol. 2012; 161:20–5.
Article9). Sedlak K, Bartova E, Machova J. Antibodies to selected viral disease agents in wild boars from the Czech Republic. J Wildl Dis. 2008; 44:777–80.
Article10). Montagnaro S, Sasso S, De Martino L, Longo M, Iovane V, Ghiurmino G, et al. Prevalence of antibodies to selected viral and bacterial pathogens in wild boar (Sus scrofa) in Campania Region, Italy. J Wildl Dis. 2010; 46:316–9.11). Cramer SD, Campbell GA, Njaa BL, Morgan SE, Smith SK 2nd, McLin WR 4th, et al. Pseudorabies virus infection in Oklahoma hunting dogs. J Vet Diagn Invest. 2011; 23:915–23.
Article12). OIE (Office International des epizooties). 2012 Aujeszky's disease. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees). 7th ed.Paris: OIE;;2012. p. 97–111.13). Vicente J, León-Vizcaíno L, Gortázar C, José Cubero M, González M, Martín-Atance P. Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain. J Wildl Dis. 2002; 38:649–52.
Article14). Martino PE, Montenegro JL, Preziosi JA, Venturini C, Bacigalupe D, Stanchi NO, et al. Serological survey of selected pathogens of free-ranging foxes in southern Argentina, 1998–2001. Rev Sci Tech. 2004; 23:801–6.
Article15). Ruiz-Fons F, Vidal D, Höfle U, Vicente J, Gortázar C. Aujeszky's disease virus infection patterns in European wild boar. Vet Microbiol. 2007; 120:241–50.
Article16). Platt KB, Graham DL, Faaborg RA. Pseudorabies: experimental studies in raccoons with different virus strains. J Wildl Dis. 1983; 19:297–301.
Article17). Weigel RM, Hahn EC, Scherba G. Survival and immunization of raccoons after exposure to pseudorabies (Aujeszky's disease) virus gene-deleted vaccines. Vet Microbiol. 2003; 92:19–24.
Article18). Elbers AR, Tielen MJ, Cromwijk WA, Hunneman WA. Sero-epidemiological screening of pig sera collected at the slaughterhouse to detect herds infected with Aujeszky's disease virus, porcine influenza virus and Actinobacillus (Haemophilus) pleuropneumoniae in the framework of an integrated quality control (IQC) system. Vet Q. 1990; 12:221–30.19). Pensaert M, Labarque G, Favoreel H, Nauwynck H. Aujeszky's disease vaccination and differentiation of vaccinated from infected pigs. Dev Biol (Basel). 2004; 119:243–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serologic Survey of Rabies Virus, Canine Distemper Virus and Parvovirus in Wild Raccoon Dogs (Nyctereutes procyonoides koreensis) in Korea
- Detection of viral infections in wild Korean raccoon dogs (Nyctereutes procyonoides koreensis)
- Effects of alprazolam on capture stress-related serum cortisol responses in Korean raccoon dogs (Nyctereutes procyonoides koreensis)
- New Definitive Hosts and Differential Body Indices of Isthmiophora hortensis (Digenea: Echinostomatidae)
- Radiographic evaluation of congenital vertebral anomalies in Korean raccoon dogs (Nyctereutes procyonoides koreensis)