Korean J Lab Med.
2011 Jul;31(3):212-218. 10.3343/kjlm.2011.31.3.212.
Individual Variation in Growth Factor Concentrations in Platelet-rich Plasma and Its Influence on Human Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Yeungnam University, Daegu, Korea.
- 2Department of Anatomy, College of Medicine, Yeungnam University, Daegu, Korea.
- 3Department of Physiology, College of Medicine, Yeungnam University, Daegu, Korea.
- 4Department of Orthopedic Surgery, College of Medicine, Yeungnam University, Daegu, Korea. mwahn@ynu.ac.kr
- 5Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 1735857
- DOI: http://doi.org/10.3343/kjlm.2011.31.3.212
Abstract
- BACKGROUND
The objective of this study was to explore whether individual variations in the concentration of growth factors (GFs) influence the biologic effects of platelet-rich plasma (PRP) on human mesenchymal stem cells (HMSCs).
METHODS
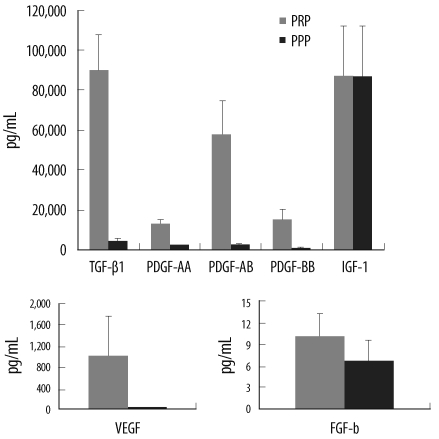
The concentrations of 7 representative GFs in activated PRP (aPRP) were measured using ELISA. The effects of PRP on the proliferation and alkaline phosphatase (ALP) activity of HMSCs were examined using several concentrations of aPRP from 3 donors; the relationships between the GF levels and these biologic effects were then evaluated using 10% aPRP from 5 subgroups derived from 39 total donors. HMSCs were cultured in DMEM with the addition of aPRP for 4 or 12 days; then, DNA content and ALP activity were measured.
RESULTS
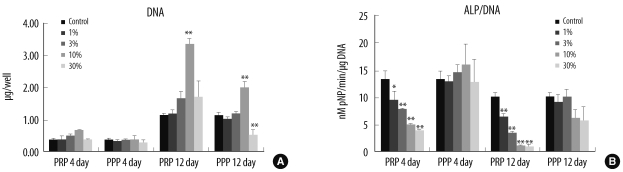
The quantity of DNA increased significantly at a 10% concentration of aPRP, but the ALP activity was suppressed at this concentration of aPRP. The GF concentrations varied among donors, and 5 subgroups of characteristic GF release patterns were identified via cluster analysis. DNA levels differed significantly between groups and tended to be higher in groups with higher concentrations of transforming growth factor-beta1 (TGF-beta1) and platelet-derived growth factors (PDGFs). DNA quantity was positively correlated with TGF-beta1 concentration, and was negatively correlated with donor age. ALP activity was negatively correlated with PDGF-BB concentration.
CONCLUSIONS
The varying GF concentrations may result in different biologic effects; thus, individual differences in GF levels should be considered for reliable interpretation of the biologic functions and standardized application of PRP.
Keyword
MeSH Terms
-
Alkaline Phosphatase/metabolism
Blood Donors
Cell Differentiation
Cells, Cultured
Culture Media/chemistry
DNA/analysis
Humans
Intercellular Signaling Peptides and Proteins/*pharmacology
Mesenchymal Stem Cells/*cytology/drug effects
Platelet-Derived Growth Factor/pharmacology
Platelet-Rich Plasma/*metabolism
Transforming Growth Factor beta1/pharmacology
Figure
Cited by 2 articles
-
Synergistic Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma in Streptozotocin-Induced Diabetic Rats
Zhenzhen Lian, Xiaojing Yin, Hua Li, Lili Jia, Xiuzhen He, Yongbo Yan, Naihua Liu, Kayiu Wan, Xiaokun Li, Shaoqiang Lin
Ann Dermatol. 2014;26(1):1-10. doi: 10.5021/ad.2014.26.1.1.Effects of Platelet Lysate Preparations on the Proliferation of HaCaT Cells
Sae Yun Baik, Young Ae Lim, Seon Joo Kang, Sun Hyun Ahn, Wee Gyo Lee, Chul Ho Kim
Ann Lab Med. 2014;34(1):43-50. doi: 10.3343/alm.2014.34.1.43.
Reference
-
1. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004; 91:4–15. PMID: 14691563.
Article2. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 114:1502–1508. PMID: 15509939.
Article3. Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009; 91:987–996. PMID: 19651823.4. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:638–646. PMID: 9638695.5. Lucarelli E, Fini M, Beccheroni A, Giavaresi G, Di Bella C, Aldini NN, et al. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res. 2005; 435:62–68. PMID: 15930922.
Article6. Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002; 22:45–53. PMID: 11922217.7. Ranly DM, McMillan J, Keller T, Lohmann CH, Meunch T, Cochran DL, et al. Platelet-derived growth factor inhibits demineralized bone matrix-induced intramuscular cartilage and bone formation. A study of immunocompromised mice. J Bone Joint Surg Am. 2005; 87:2052–2064. PMID: 16140821.8. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009; 37:2259–2272. PMID: 19875361.9. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002; 30:97–102. PMID: 12069512.
Article10. Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007; 18:639–648. PMID: 17590158.
Article11. Lennon DP, Haynesworth SE, Arm DM, Baber MA, Caplan AI. Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dyn. 2000; 219:50–62. PMID: 10974671.
Article12. Song IH, Caplan AI, Dennis JE. In vitro dexamethasone pretreatment enhances bone formation of human mesenchymal stem cells in vivo. J Orthop Res. 2009; 27:916–921. PMID: 19137580.
Article13. Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009; 15:431–435. PMID: 19216642.
Article14. Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB, et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004; 15:29–35. PMID: 14985174.15. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006; 17:212–219. PMID: 16584418.
Article16. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008; 122:1352–1360. PMID: 18971718.
Article17. Hsu CW, Yuan K, Tseng CC. The negative effect of platelet-rich plasma on the growth of human cells is associated with secreted thrombospondin-1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:185–192. PMID: 18805712.
Article18. Anitua E, Andia I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005; 23:281–286. PMID: 15779147.
Article19. Arpornmaeklong P, Kochel M, Depprich R, Kübler NR, Würzler KK. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004; 33:60–70. PMID: 14690661.
Article20. Schmidmaier G, Herrmann S, Green J, Weber T, Scharfenberger A, Haas NP, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006; 39:1156–1163. PMID: 16863704.
Article21. Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008; 92:161–168. PMID: 19043663.
Article22. Cassiede P, Dennis JE, Ma F, Caplan AI. Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res. 1996; 11:1264–1273. PMID: 8864901.23. Kim SJ, Kim SY, Kwon CH, Kim YK. Differential effect of FGF and PDGF on cell proliferation and migration in osteoblastic cell. Growth Factors. 2007; 25:77–86. PMID: 17852407.24. Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C, Ishizawa S, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008; 23:1519–1528. PMID: 18410236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Safety of Platelet-Rich Plasma and Adipose-Derived Stem Cells: An Update
- Use of Platelet-rich Plasma
- Platelet-rich plasma, platelet derivatives, and their therapeutic importance in veterinary medicine
- Acceleration of Wound Healing Using Adipose-derived Stem Cell Therapy with Platelet Concentrates: Platelet-rich Plasma (PRP) vs. Platelet-rich Fibrin (PRF)
- Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix