Yonsei Med J.
2005 Jun;46(3):399-407. 10.3349/ymj.2005.46.3.399.
Comparison of Rifaximin and Lactulose for the Treatment of Hepatic Encephalopathy: A Prospective Randomized Study
- Affiliations
-
- 1Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea. leeks519@yumc.yonsei.ac.kr
- KMID: 1734076
- DOI: http://doi.org/10.3349/ymj.2005.46.3.399
Abstract
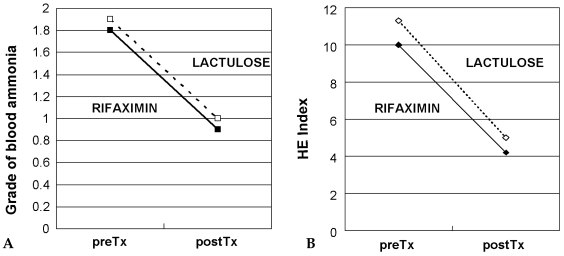
- Rifaximin has been reported to be effective for the treatment of hepatic encephalopathy (HE) in Europe. However, it is unknown whether Rifaximin is effective for the treatment of HE in Koreans, therefore we conducted a open-label prospective randomized study to evaluate the efficacy of rifaximin versus lactulose in Korean patients. Fifty-four patients with liver cirrhosis and hepatic encephalopathy were enrolled. Thirty-two patients were randomized to receive rifaximin and 22 to receive lactulose both over a 7-day periods. Before and at the end of treatment, gradation of blood ammonia, flapping tremor, mental status, number connection test (NCT) were performed and estimation of HE indexes determined. Both rifaximin and lactulose were effective in the majority of patients (84.4% and 95.4%, respectively, p=0.315). Blood NH3, flapping tremor, mental status, and NCT was significantly improved by rifaximin and lactulose, and the post- treatment levels of these measures were similar for the rifaximin and lactulose-treated groups, as was the HE index (rifaximin group (10.0-->> 4.2, p=0.000) ; lactulose group (11.3-->> 5.0, p=0.000) ). One patient treated with rifaximin complained of abdominal pain, which was easily controlled. There was no episode of renal function impairment in either treatment group. Rifaximin proved to be as safe and as effective as lactulose for the treatment of Korean patients with hepatic encephalopathy.
Keyword
MeSH Terms
-
Comparative Study
Female
Gastrointestinal Agents/*administration & dosage/adverse effects
Hepatic Encephalopathy/*drug therapy
Humans
Lactulose/*administration & dosage/adverse effects
Male
Middle Aged
Prospective Studies
Research Support, Non-U.S. Gov't
Rifamycins/*administration & dosage/adverse effects
Treatment Outcome
Figure
Reference
-
1. Lizardi-Cervera J, Almeda P, Guevara L, Uribe M. Hepatic encephalopathy: a review. Ann Hepatol. 2003; 2:122–130. PMID: 15115963.
Article2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997; 337:473–479. PMID: 9250851.
Article3. Bosch J, Bruix J, Mas A, Navasa M, Rodes J. Rolling review: the treatment of major complications of cirrhosis. Aliment Pharmacol Ther. 1994; 8:639–657. PMID: 7696453.4. Clausen MR, Mortensen PB. Lactulose, disaccharides and colonic flora. Clinical consequences. Drugs. 1997; 53:930–942. PMID: 9179525.5. Weber FL Jr. Lactulose and combination therapy of hepatic encephalopathy: the role of the intestinal microflora. Dig Dis. 1996; 14(Suppl 1):53–63. PMID: 8872452.
Article6. Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995; 49:467–484. PMID: 7774516.7. Testa R, Eftimiadi C, Sukkar GS, De Leo C, Rovida S, Schito GC, et al. A non-absorbable rifamycin for treatment of hepatic encephalopathy. Drugs Exp Clin Res. 1985; 11:387–392. PMID: 3836135.8. Di Piazza S, Gabriella Filippazzo M, Valenza LM, Morello S, Pastore L, Conti A, et al. Rifaximine versus neomycin in the treatment of portosystemic encephalopathy. Ital J Gastroenterol. 1991; 23:403–407. PMID: 1742538.9. Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol. 1991; 23:175–178. PMID: 1751811.10. Festi D, Mazzella G, Parini P, Ronchi M, Cipolla A, Orsini M, et al. Treatment of hepatic encephalopathy with non-absorbable antibiotics. Ital J Gastroenterol. 1992; 24:14–16. PMID: 1486194.11. Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin. 1993; 13:109–118. PMID: 8325041.12. Miglio F, Valpiani D, Rossellini SR, Ferrieri A. Rifaximin, a non-absorbable rifamycin, for the treatment of hepatic encephalopathy. A double-blind, randomised trial. Curr Med Res Opin. 1997; 13:593–601. PMID: 9327194.
Article13. Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000; 12:203–208. PMID: 10741936.14. Mas A, Rodes J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003; 38:51–58. PMID: 12480560.
Article15. Reddy KR, Hoofnagle JH, Tong MJ, Lee WM, Pockros P, Heathcote EJ, et al. Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology. 1999; 30:787–793. PMID: 10462387.16. Nguyen MH, Whittemore AS, Garcia RT, Tawfeek SA, Ning J, Lam S, et al. Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol. 2004; 2:820–824. PMID: 15354283.
Article17. Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002; 359:287–293. PMID: 11830194.
Article18. Huh K, Choi SY, Whang YS, Lee DS. Prevalence of viral hepatitis markers in Korean patients with hepatocellular carcinoma. J Korean Med Sci. 1998; 13:306–310. PMID: 9681811.
Article19. Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004; 24:217–232. PMID: 15349801.
Article20. Morencos FC, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995; 40:1252–1256. PMID: 7781442.21. Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977; 72:573–583. PMID: 14049.22. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955; 19:393–394. PMID: 13263471.
Article23. Conn HO. Trailmaking and number-connection tests in the assessment of mental state in portal systemic encephalopathy. Am J Dig Dis. 1977; 22:541–550. PMID: 868833.
Article24. Elkington SG, Floch MH, Conn HO. Control of chronic portal-systemic encephalopathy by lactulose. Gut. 1969; 10:416. PMID: 4890540.25. Simmons F, Goldstein H, Boyle JD. A controlled clinical trial of lactulose in hepatic encephalopathy. Gastroenterology. 1970; 59:827–832. PMID: 4922276.
Article26. Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, et al. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997; 26:1410–1414. PMID: 9397979.
Article27. Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004; 328:1046–1051. PMID: 15054035.
Article28. Blei AT, Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001; 96:1968–1976. PMID: 11467622.
Article29. Kircheis G, Haussinger D. Management of hepatic encephalopathy. J Gastroenterol Hepatol. 2002; 17(Suppl 3):S260–S267. PMID: 12472947.
Article30. Warren SE, Mitas JA 2nd, Swerdlin AH. Hypernatremia in hepatic failure. JAMA. 1980; 243:1257–1260. PMID: 7359682.
Article31. Loft S, Sonne J, Dossing M, Andreasen PB. Metronidazole pharmacokinetics in patients with hepatic encephalopathy. Scand J Gastroenterol. 1987; 22:117–123. PMID: 3563404.
Article32. Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994; 14:51–56. PMID: 7836025.33. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy-Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002; 35:716–721. PMID: 11870389.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Short-term Administration of Rifaximin in the Treatment of Hepatic Encephalopathy
- Osmotic Demyelination Syndrome Associated with Hypernatremia Caused by Lactulose Enema in a Patient with Chronic Alcoholism
- A Case of Pneumatosis Cystoides Intestinalis in a Cirrhosis Patient
- Use of Oral Antibiotics in Elderly Gastrointestinal Patients
- Determination of Rifaximin Treatment Period According to Lactulose Breath Test Values in Nonconstipated Irritable Bowel Syndrome Subjects