Yonsei Med J.
2005 Jun;46(3):347-352. 10.3349/ymj.2005.46.3.347.
Effectiveness of Real-Time Quantitative PCR Compare to Repeat PCR for the Diagnosis of Charcot-Marie-Tooth Type 1A and Hereditary Neuropathy with Liability to Pressure Palsies
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. jlim@yumc.yonsei.ac.kr
- 2Department of Neurology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Konkuk University College of Medicine, Seoul, Korea.
- KMID: 1734068
- DOI: http://doi.org/10.3349/ymj.2005.46.3.347
Abstract
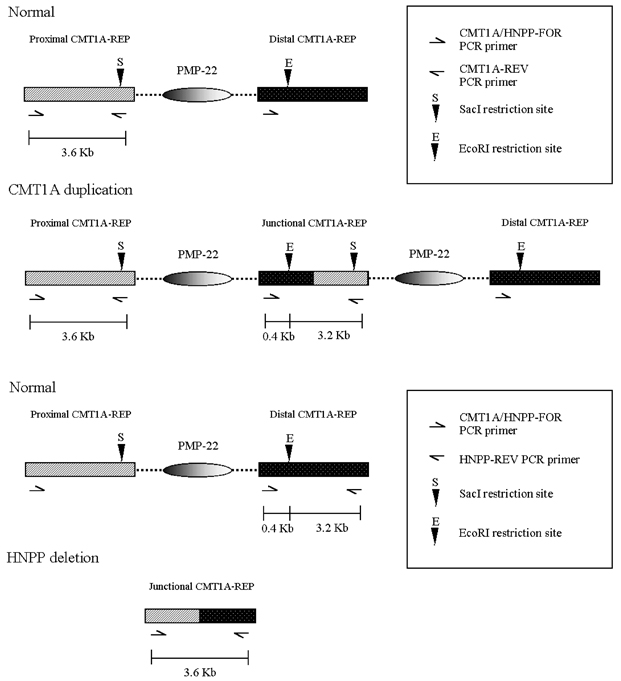
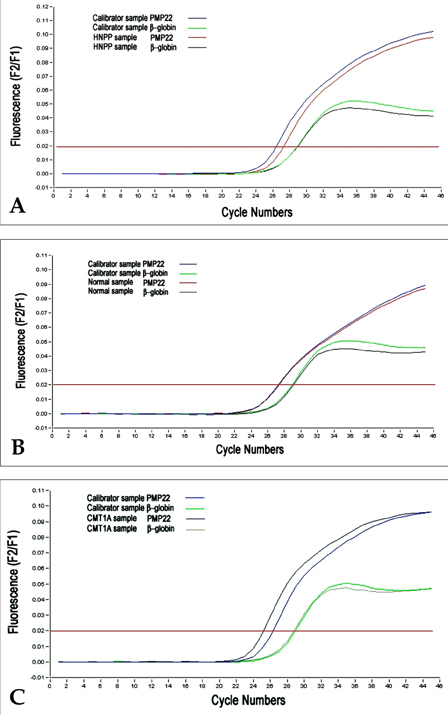
- The majority of cases of Charcot-Marie-Tooth type 1A (CMT1A) and of hereditary neuropathy with a liability to pressure palsies (HNPP) are the result of heterozygosity for the duplication or deletion of peripheral myelin protein 22 gene (PMP22) on 17p11.2. Southern blots, pulsed-field gel electrophoresis (PFGE), fluorescence in situ hybridization (FISH) and polymorphic marker analysis are currently used diagnostic methods. But they are time-consuming, labor-intensive and have some significant limitations. We describe a rapid real- time quantitative PCR method for determining gene copy number for the identification of DNA duplication or deletion occurring in CMT1A or HNPP and compare the results obtained with REP-PCR. Six patients with CMT1A and 14 patients with HNPP [confirmed by Repeat (REP) -PCR], and 16 patients with suspicious CMT1A and 13 patients with suspicious HNPP [negative REP-PCR], and 15 normal controls were studied. We performed REP-PCR, which amplified a 3.6 Kb region (including a 1.7Kb recombination hotspot), using specific CMT1A-REP and real-time quantitative PCR on the LightCycler system. Using a comparative threshold cycle (Ct) method and beta-globin as a reference gene, the gene copy number of the PMP22 gene was quantified. The PMP22 duplication ratio ranged from 1.35 to 1.74, and the PMP22 deletion ratio from 0.41 to 0.53. The PMP22 ratio in normal controls ranged from 0.81 to 1.12. All 6 patients with CMT1A and 14 patients with HNPP confirmed by REP-PCR were positive by real-time quantitative PCR. Among the 16 suspicious CMT1A and 13 suspicious HNPP with negative REP-PCR, 2 and 4 samples, respectively, were positive by real-time quantitative PCR. Real-time quantitative PCR is a more sensitive and more accurate method than REP-PCR for the detection of PMP22 duplications or deletions, and it is also faster and easier than currently available methods. Therefore, we believe that the real-time quantitative method is useful for diagnosing CMT1A and HNPP.
Keyword
MeSH Terms
Figure
Reference
-
1. Lupski JR, Garcia CA. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Charcot-Marie-Tooth peripheral neuropathies and related disorders. The metabolic & molecular bases of inherited disease. 2001. 8th ed. New York: McGraw-Hill;5759–5788.2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, Pentao L, Guzzetta V, Trask BJ, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991. 66:219–232.3. MacMillan JC, Upadhyaya M, Harper PS. Charcot-Marie-Tooth disease type 1a (CMT1a): evidence for trisomy of the region p11.2 of chromosome 17 in south Wales families. J Med Genet. 1992. 29:12–13.4. Raeymaekers P, Timmerman V, Nelis E, de Jonghe P, Hoogendijk JE, Baas F, et al. The HMSN Collaborative Research Group: Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a). Neuromuscul Disord. 1991. 1:93–97.5. Raeymaekers P, Timmerman V, Nelis E, van Hul W, de Jonghe P, Martin JJ, et al. HMSN Collaborative Research Group. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). J Med Genet. 1992. 29:5–11.6. Chen KL, Wang YL, Dodson LA, Rennert H, Mochan BS, Wilson R, et al. Normalized Southern hybridization to enhance testing for Charcot-Marie-Tooth disease, type 1A. Mol Diagn. 1996. 1:65–71.7. Hoogendijk JE, Hensels GW, Gabreels-Festen AA, Gabreels FJ, Janssen EA, de Jonghe P, et al. De novo mutation in hereditary motor and sensory neuropathy type I. Lancet. 1992. 339:1081–1082.8. Hoogendijk JE, Janssen EA, Gabreels-Festen AA, Hensels GW, Joosten EM, Gabreels FJ, et al. Allelic heterogeneity in hereditary motor and sensory neuropathy type Ia (Charcot-Marie-Tooth disease type 1A). Neurology. 1993. 43:1010–1015.9. Ikegami T, Ikeda H, Chance PF, Kiyosawa H, Yamamoto M, Sobue G, et al. Facilitated diagnosis of CMT1A duplication in chromosome 17p11.2-12: analysis with a CMT1A-REP repeat probe and photostimulated luminescence imaging. Hum Mutat. 1997. 9:563–566.10. Chance PF, Abbas N, Lensch MW, Pentao L, Roa BB, Patel PI, et al. Two autosomal dominant neuropathies result from reciprocal DNA duplication/deletion of a region on chromosome 17. Hum Mol Genet. 1994. 3:223–228.11. Pentao L, Wise CA, Chinault AC, Patel PI, Lupski JR. Charcot-Marie-Tooth type 1A duplication appears to arise from recombination at repeat sequences flanking the 1.5-Mb monomer unit. Nat Genet. 1992. 2:292–300.12. Timmerman V, Lofgren A, le Guern E, Liang P, de Jonghe P, Martin JJ, et al. Molecular genetic analysis of the17p11.2 region in patients with hereditary neuropathy with liability to pressure palsies (HNPP). Hum Genet. 1996. 97:26–34.13. Roa BB, Greenberg F, Gunaratne P, Sauer CM, Lubinsky MS, Kozma C, et al. Duplication of the PMP 22 gene in 17p partial trisomy patients with Charcot-Marie-Tooth type-1 neuropathy. Hum Genet. 1996. 97:642–649.14. Shaffer LG, Kennedy GM, Spikes AS, Lupski JR. Diagnosis of CMT1A duplications and HNPP deletions by interphase FISH: implications for testing in the cytogenetics laboratory. Am J Med Genet. 1997. 69:325–331.15. Navon R, Timmerman V, Lofgren A, Liang P, Nelis E, Zeitune M, et al. Prenatal diagnosis of Charcot-Marie-Tooth disease type 1A (CMT1A) using molecular genetic techniques. Prenat Diagn. 1995. 15:633–640.16. Haupt A, Schols L, Przuntek H, Epplen JT. Polymorphisms in the PMP-22 gene region (17p11.2-12) are crucial for simplified diagnosis of duplications/deletions. Hum Genet. 1997. 99:688–691.17. Stronach EA, Clark C, Bell C, Lofgren A, McKay NG, Timmerman V, et al. Novel PCR-based diagnostic tools for Charcot-Marie-Tooth type 1A and hereditary neuropathy with liability to pressure palsies. J Peripher Nerv Syst. 1999. 4:117–122.18. Yamamoto M, Keller MP, Yasuda T, Hayasaka K, Ohnishi A, Yoshikawa H, et al. Clustering of CMT1A duplication breakpoints in a 700-bp interval of the CMT1A-REP repeat. Hum Mutat. 1998. 11:109–113.19. Poropat RA, Nicholson GA. Determination of gene dosage at the PMP22 and androgen receptor loci by quantitative PCR (comments). Clin Chem. 1998. 44:724–730.20. Young P, Stogbauer F, Wiebusch H, Lofgren A, Timmerman V, van Broeckhoven C, et al. PCR-based strategy for the diagnosis of hereditary neuropathy with liability to pressure palsies and Charcot-Marie-Tooth disease type 1A. Neurology. 1998. 50:760–763.21. Livak KJ. Comparative Ct method. ABI Prism 7700 Sequence Detection System. User Bulletin No. 2. 1997. PE Applied Bio-systems.22. Thiel CT, Kraus C, Rauch A, Ekici AB, Rautenstrauss B, Reis A. A new quantitative PCR multiplex assay for rapid analysis of chromosome 17p11.2-12 duplications and deletions leading to HMSN/HNPP. Eur J Hum Genet. 2003. 11:170–178.23. Lorentzos P, Kaiser T, Kennerson ML, Nicholson GA. A rapid and definitive test for Charcot-Marie-Tooth 1A and hereditary neuropathy with liability to pressure palsies using multiplexed real-time PCR. Genet Test. 2003. 7:135–138.24. Kim SW, Lee KS, Jin HS, Lee TM, Koo SK, Lee YJ, et al. Rapid Detection of duplication/deletion of the PMP 22 gene in patients with Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsy by real-time quantitative PCR using SYBR Green I Dye. J Korean Med Sci. 2003. 18:727–732.25. Kim YR, Choi JR, Song KS, Chong WH, Lee HD. Evaluation of HER2/neu status by real-time quantitative PCR in breast cancer. Yonsei Med J. 2002. 43:335–340.26. Nelis E, Haites N, Van Broeckhoven C. Mutations in the peripheral myelin genes and associated genes in inherited peripheral neuropathies. Hum Mutat. 1999. 13:11–28.27. De Visser M, Van Broeckhoven C, Nelis E. Hereditary motor and sensory neuropathy or Charcot-Marie-Tooth disease type 1A and 1B. 1997. 2nd ed. London: Royal Soc Med, London/Eur Neuromuscular Centre, Royal Society of Medicine Press;49–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Family of Hereditary Neuropathy with Liability to Pressure Palsy Presenting Atypical Electrophysiological Features
- Rapid Detection of Duplication/Deletion of the PMP22 Gene in Patients with Charcot-Marie-Tooth Disease Type 1A and Hereditary Neuropathy with Liability to Pressure Palsy by Real-time Quantitative PCR using SYBR Green I Dye
- Current Issues of the Charcot-Marie-Tooth Disease
- A Family Harboring CMT1A Duplication and HNPP Deletion
- Charcot-Marie-Tooth 1A Concurrent with Schwannomas of the Spinal Cord and Median Nerve