J Korean Med Sci.
2004 Apr;19(2):258-262. 10.3346/jkms.2004.19.2.258.
X-chromosome Inactivation Patterns in Korean Women with Idiopathic Recurrent Spontaneous Abortion
- Affiliations
-
- 1Laboratory of Medical Genetics, Samsung Cheil Hospital and Women's Healthcare Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ryu97@samsung.co.kr
- 2Department of Obstetrics and Gynecology, Samsung Cheil Hospital and Women's Healthcare Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1733486
- DOI: http://doi.org/10.3346/jkms.2004.19.2.258
Abstract
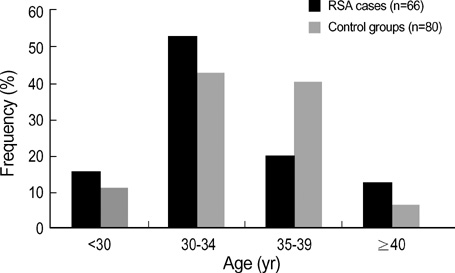
- Recurrent spontaneous abortion (RSA) defines as two or more consecutive losses at < or =20 weeks of gestation and affects an estimated 1 of every 100 couples wishing to have children. However, it remains a poorly understood phenomenon. Recent reports observed a significant association between highly skewed X chromosome and RSA, supporting that X chromosome inactivation might be an important and previously unknown cause of RSA. X-inactivation pattern, using polymeric X-linked women with idiopathic RSA and 80 control subjects with a single successful pregnancy and no history of spontaneous abortion. The ratio of heterozygotes was 68.2% (45/66) in women with RSA and 67.5% (54/80) in control group. Among 45 informative RSA cases, only 1 (2.2%) woman showed extreme skewed X inactivation (> or =90%) and 4 (8.9%) had mild skewed inactivation (> or =85%). In 54 heterozygous control subjects, 5 (9.3%) women showed extreme skewed X inactivation and 7 (13.0%) had mild one. The frequency of skewed X inactivation between RSA patients and control group was not significantly different (p>0.05). This finding suggests that skewed x romosome be not associated with unexplained RSA patients.
MeSH Terms
Figure
Reference
-
1. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996. 66:24–29.2. Hatasaka HH. Recurrent miscarriage: epidemiologic factors, definitions, and incidence. Clin Obstet Gynecol. 1994. 37:625–634.
Article3. Sangha KK, Stephenson MD, Brown CJ, Robinson WP. Extremely skewed X-chromosome inactivation is increased in women with recurrent spontaneous abortion. Am J Hum Genet. 1999. 65:913–917.
Article4. Lanasa MC, Hogge WA, Kubik C, Blancato J, Hoffman EP. Highly skewed X-chromosome inactivation is associated with idiopathic recurrent spontaneous abortion. Am J Hum Genet. 1999. 65:252–254.
Article5. Uehara S, Hashiyada M, Sato K, Sato Y, Fujimori K, Okamura K. Preferential X-chromosome inactivation in women with idiopathic recurrent pregnancy loss. Fertil Steril. 2001. 76:908–914.
Article6. Lanasa MC, Hogge WA, Kubik CJ, Ness RB, Harger J, Nagel T, Prosen T, Markovic N, Hoffman EP. A novel X chromosome-linked genetic cause of recurrent spontaneous abortion. Am J Obstet Gynecol. 2001. 185:563–568.
Article7. Wengler G, Gorlin JB, Williamson JM, Rosen FS, Bing DH. Nonrandom inactivation of the X chromosome in early lineage hematopoietic cells in carriers of Wiskott-Aldrich syndrome. Blood. 1995. 85:2471–2477.
Article8. Naumova AK, Plenge RM, Bird LM, Leppert M, Morgan K, Willard HF, Sapienza C. Heritability of X chromosome-inactivation phenotype in a large family. Am J Hum Genet. 1996. 58:1111–1119.9. Devriendt K, Matthijs G, Legius E, Schollen E, Blockmans D, van Geet C, Degreef H, Cassiman JJ, Fryns JP. Skewed X-chromosome inactivation in female carriers of dyskeratosis congenita. Am J Hum Genet. 1997. 60:581–587.10. Pegoraro E, Whitaker J, Mowery-Rushton P, Surti U, Lanasa M, Hoffman EP. Familial skewed X inactivation: a molecular trait associated with high spontaneous-abortion rate maps to Xq28. Am J Hum Genet. 1997. 61:160–170.
Article11. Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992. 51:1229–1239.12. Green AJ, Sepp T, Yates JR. Clonality of tuberous sclerosis harmatomas shown by non-random X-chromosome inactivation. Hum Genet. 1996. 97:240–243.
Article13. Lau AW, Brown CJ, Penaherrera M, Langlois S, Kalousek DK, Robinson WP. Skewed X-chromosome inactivation is common in fetuses or newborns associated with confined placental mosaicism. Am J Hum Genet. 1997. 61:1353–1361.
Article14. Beever CL, Stephenson MD, Penaherrera MS, Jiang RH, Kalousek DK, Hayden M, Field L, Brown CJ, Robinson WP. Skewed X-chromosome inactivation is associated with trisomy in women ascertained on the basis of recurrent spontaneous abortion or chromosomally abnormal pregnancies. Am J Hum Genet. 2003. 72:399–407.
Article15. Busque L, Mio R, Mattioli J, Brais E, Blais N, Lalonde Y, Maragh M, Gilliland DG. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood. 1996. 88:59–65.
Article16. Kopp P, Jaggi R, Tobler A, Borisch B, Oestreicher M, Sabacan L, Jameson JL, Fey MF. Clonal X-inactivation analysis of human tumours using the human androgen receptor gene (HUMARA) polymorphism: a non-radioactive and semiquantitative strategy applicable to fresh and archival tissue. Mol Cell Probes. 1997. 11:217–228.
Article17. Sharp A, Robinson D, Jacobs P. Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet. 2000. 107:343–349.
Article18. Plenge RM, Stevenson RA, Lubs HA, Schwartz CE, Willard HF. Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am J Hum Genet. 2002. 71:168–173.
Article19. Plenge RM, Hendrich BD, Schwartz C, Arena JF, Naumova A, Sapienza C, Winter RM, Willard HF. A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet. 1997. 17:353–356.
Article20. Gale RE, Fielding AK, Harrison CN, Linch DC. Acquired skewing of X-chromosome inactivation patterns in myeloid cells of the elderly suggests stochastic clonal loss with age. Br J Haematol. 1997. 98:512–519.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Cytogenetic Study of 130 Couples with Recurrent Spontaneous Abortion
- Chromosome analysis from chorionic villi samples in recurrent spontaneous abortion
- A Cytogenetic Study of Recurrent Spontaneous Abortion
- Cytogenetic abnormalities in patients with reproductive dysfunction
- The Analysis of Methylenetetrahydrofolate Reductase Mutation in Recurrent Spontaneous Abortion Associated with Hyperhomocysteinemia