Yonsei Med J.
2011 Jul;52(4):695-698. 10.3349/ymj.2011.52.4.695.
Bowel Perforation after Erlotinib Treatment in a Patient with Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Gyeongsang National University, Jinju, Korea. brightree@lycos.co.kr
- 2Gyeongsang Institute of Health Science, Jinju, Korea.
- 3Gyeongnam Regional Cancer Center, Jinju, Korea.
- KMID: 1728007
- DOI: http://doi.org/10.3349/ymj.2011.52.4.695
Abstract
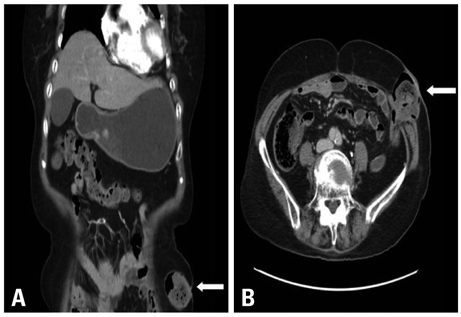
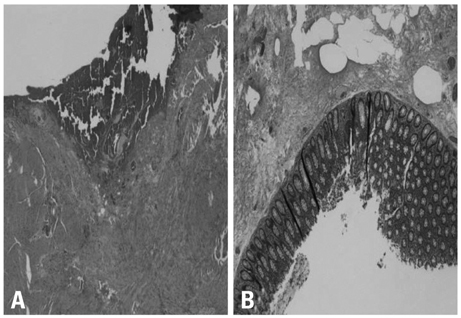
- Erlotinib is accepted as a standard second-line chemotherapeutic agent in patients with non-small cell lung cancer who are refractory or resistant to first-line platinum-based chemotherapy. There has been no previous report of bowel perforation with or without gastrointestinal metastases related to erlotinib in patients with non-small cell lung cancer. The exact mechanism of bowel perforation in patients who received erlotinib remains unclear. In this report, we report the first case of enterocutaneous fistula in a female patient with metastatic non-small cell lung cancer 9 months, following medication with erlotinib as second-line chemotherapy.
Keyword
MeSH Terms
-
Aged
Antineoplastic Agents/*adverse effects/therapeutic use
Carcinoma, Non-Small-Cell Lung/complications/*drug therapy
Female
Humans
Intestinal Fistula/*chemically induced/complications/radiography/surgery
Intestinal Perforation/*chemically induced/complications/radiography/surgery
Protein Kinase Inhibitors/*adverse effects/therapeutic use
Quinazolines/*adverse effects/therapeutic use
Sigmoid Diseases/*chemically induced/complications/radiography/surgery
Figure
Reference
-
1. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2010. CD007309.2. Uhm JE, Park BB, Ahn MJ, Lee J, Ahn JS, Kim SW, et al. Erlotinib monotherapy for stage IIIB/IV non-small cell lung cancer: a multicenter trial by the Korean Cancer Study Group. J Thorac Oncol. 2009. 4:1136–1143.
Article3. Dudek AZ, Kmak KL, Koopmeiners J, Keshtgarpour M. Skin rash and bronchoalveolar histology correlates with clinical benefit in patients treated with gefitinib as a therapy for previously treated advanced or metastatic non-small cell lung cancer. Lung Cancer. 2006. 51:89–96.
Article4. Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP. Skin rash and good performance status predict improved survival with gefitinib in patients with advanced non-small cell lung cancer. Ann Oncol. 2005. 16:780–785.
Article5. Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004. 46:255–261.
Article6. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003. 290:2149–2158.
Article7. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009. 27:1227–1234.
Article8. Di Costanzo F, Mazzoni F, Micol Mela M, Antonuzzo L, Checcacci D, Saggese M, et al. Bevacizumab in non-small cell lung cancer. Drugs. 2008. 68:737–746.
Article9. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004. 22:2184–2191.
Article10. Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007. 12:356–361.
Article11. Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007. 14:1860–1869.
Article12. Johnson JR, Cohen M, Sridhara R, Chen YF, Williams GM, Duan J, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005. 11:6414–6421.
Article13. Schellhaas E, Loddenkemper C, Schmittel A, Buhr HJ, Pohlen U. Bowel perforation in non-small cell lung cancer after bevacizumab therapy. Invest New Drugs. 2009. 27:184–187.
Article14. Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008. 19:577–582.
Article15. Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003. 9:1200–1210.16. de Jong JS, van Diest PJ, van der Valk P, Baak JP. Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol. 1998. 184:53–57.
Article17. Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997. 151:1523–1530.18. Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, et al. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000. 6:1936–1948.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib
- Recurrent Erlotinib-Induced Interstitial Lung Disease on Non-Small Cell Lung Cancer
- Erlotinib HCL (Tarceva(R)) Induced Radiation Recall Dermatitis
- A Case of Acneiform Eruption and Paronychia Occurring after Use of Erlotinib (Tarceva(R))
- Erlotinib-Related Spontaneous Pneumothorax in Patient with Primary Lung Cancer