Tuberc Respir Dis.
2009 Nov;67(5):445-448.
Recurrent Erlotinib-Induced Interstitial Lung Disease on Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea. smkwak@inha.ac.kr
- 2Department of Pathology, Inha University College of Medicine, Incheon, Korea.
Abstract
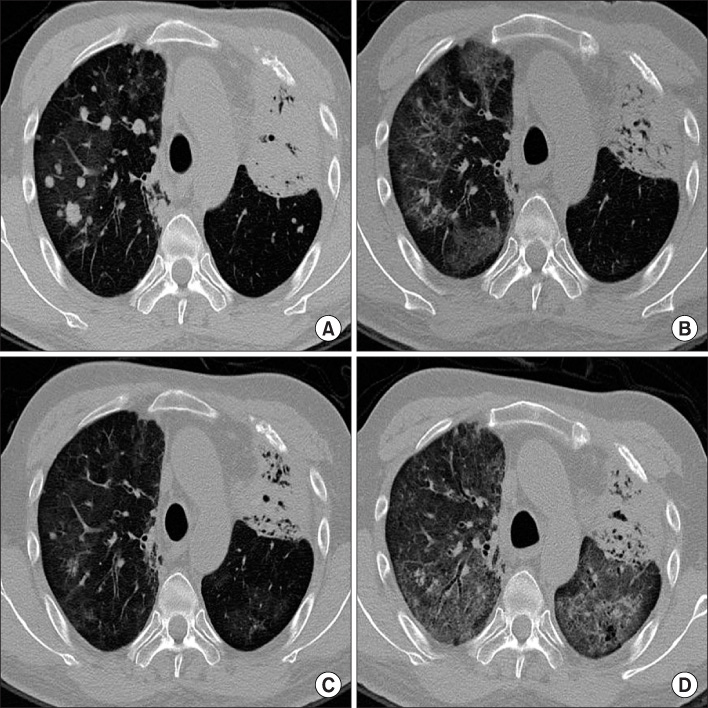
- Erlotinib (Tarceva(R)) has been considered to be a new, promising oral chemotherapy agent for local advanced or metastatic non-small cell lung cancer (NSCLC). Erlotinib is regarded as relatively safe, but interstitial lung disease (ILD) related to erlotinib has been reported on an infrequent basis in Asia. We report an histologically confirmed case of recurrent erlotinib-induced ILD. Although, the patient was highly responsive to the first erlotinib treatment, the therapy was discontinued due to erlotinib-induced ILD. After intravenous high dose methylpredinisolone treatment, ILD was improved rapidly by radiologic studies, but the particular lung cancer re-emerged. We restarted the patient erlotinib on low-dose oral methylpredinisolone, resulting in a recurrence of erlotinib-induced ILD. Our case suggests that re-administration of erlotinib should be performed on a limited basis in patients that have developed ILD on previous use, even if a therapeutic effect can be estimated.
MeSH Terms
Figure
Reference
-
1. Johnson JR, Cohen M, Sridhara R, Chen Y, Williams GM, Duan J, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005. 11:6414–6421.2. Spigel DR, Lin M, O'Neill V, Hainsworth JD. Final survival and safety results from a multicenter, open-label, phase 3b trial of erlotinib in patients with advanced nonsmall cell lung cancer. Cancer. 2008. 112:2749–2755.3. Kato T, Nishio K. Clinical aspects of epidermal growth factor receptor inhibitors: benefit and risk. Respirology. 2006. 11:693–698.4. Niho S, Kubota K, Goto K, Yoh K, Ohmatsu H, Kakinuma R, et al. First-line single agent treatment with gefitinib in patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 2006. 24:64–69.5. Fukui T, Mitsudomi T. Mutations in the epidermal growth factor receptor gene and effects of EGFR-tyrosine kinase inhibitors on lung cancers. Gen Thorac Cardiovasc Surg. 2008. 56:97–103.6. Liu V, White DA, Zakowski MF, Travis W, Kris MG, Ginsberg MS, et al. Pulmonary toxicity associated with erlotinib. Chest. 2007. 132:1042–1044.7. Yoneda KY, Shelton DK, Beckett LA, Gandara DR. Independent review of interstitial lung disease associated with death in TRIBUTE (paclitaxel and carboplatin with or without concurrent erlotinib) in advanced non-small cell lung cancer. J Thorac Oncol. 2007. 2:537–543.8. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003. 8:303–306.9. Makris D, Scherpereel A, Copin MC, Colin G, Brun L, Lafitte JJ, et al. Fatal interstitial lung disease associated with oral erlotinib therapy for lung cancer. BMC Cancer. 2007. 7:150.10. Kitajima H, Takahashi H, Harada K, Kanai A, Inomata S, Taniguchi H, et al. Gefitinib-induced interstitial lung disease showing improvement after cessation: disassociation of serum markers. Respirology. 2006. 11:217–220.11. Suzuki M, Asahina H, Konishi J, Yamazaki K, Nishimura M. Recurrent gefitinib-induced interstitial lung disease. Intern Med. 2008. 47:533–536.12. Niho S, Goto K, Yoh K, Kim YH, Ohmatsu H, Kubota K, et al. Interstitial shadow on chest CT is associated with the onset of interstitial lung disease caused by chemotherapeutic drugs. Jpn J Clin Oncol. 2006. 36:269–273.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib
- Erlotinib HCL (Tarceva(R)) Induced Radiation Recall Dermatitis
- Bowel Perforation after Erlotinib Treatment in a Patient with Non-Small Cell Lung Cancer
- A Case of Acneiform Eruption and Paronychia Occurring after Use of Erlotinib (Tarceva(R))
- A Case of Non-Small Cell Lung Cancer in a Respiratory Bronchiolitis Associated Interstitial Lung Disease Patient