Yonsei Med J.
2011 Jul;52(4):635-642. 10.3349/ymj.2011.52.4.635.
Inflammatory and Tumor Stimulating Responses after Laparoscopic Sigmoidectomy
- Affiliations
-
- 1Department of Surgery, Chungnam National University Hospital, Daejeon, Korea.
- 2Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. namkyuk@yuhs.ac
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1727997
- DOI: http://doi.org/10.3349/ymj.2011.52.4.635
Abstract
- PURPOSE
Laparoscopic colectomy has clinical benefits such as short hospital stay, less postoperative pain, and early return of bowel function. However, objective evidence of its immunologic and oncologic benefits is scarce. We compared functional recovery after open versus laparoscopic sigmoidectomy and investigated the effect of open versus laparoscopic surgery on acute inflammation as well as tumor stimulation.
MATERIALS AND METHODS
A total of 57 patients who were diagnosed with sigmoid colon cancer were randomized for elective conventional or laparoscopically assisted sigmoidectomy. Serum samples were obtained preoperatively and on postoperative day 1. C-reactive protein (CRP) and interleukin-6 (IL-6) were measured as inflammation markers, and vascular endothelial growth factor (VEGF) and insulin-like growth factor binding protein-3 (IGFBP-3) were used as tumor stimulation factors. Clinical parameters and serum markers were compared.
RESULTS
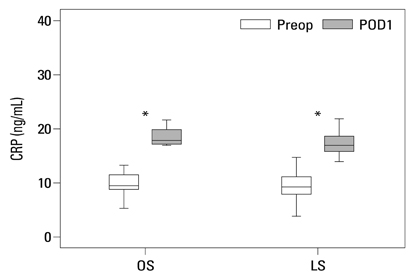
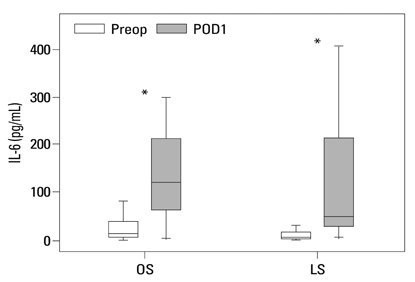
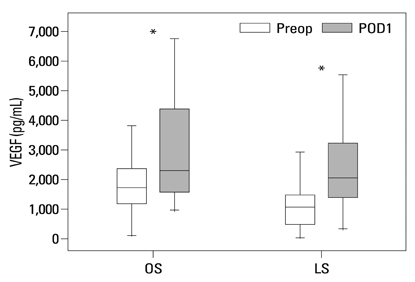
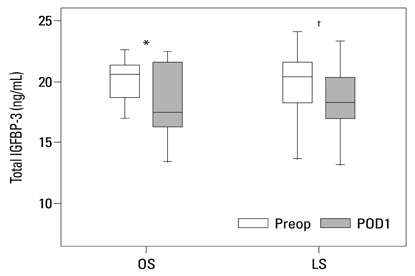
Postoperative hospital stay (p=0.031), the first day of gas out (p=0.016), and the first day of soft diet (p<0.001) were significantly shorter for the laparoscopic surgery group than the open surgery group. The levels of CRP, IL-6, and VEGF rose significantly, and the concentration of IGFBP-3 fell significantly after both open and laparoscopic surgery. However, there were no significant differences in the preoperative and postoperative levels of CRP, IL-6, VEGF, and IGFBP-3 between the two groups.
CONCLUSION
Our data suggest that both open and laparoscopic surgeries are accompanied by significant changes in IL-6, CRP, IGFBP-3, and VEGF levels. Acute inflammation markers and tumor stimulating factors may not reflect clinical benefits of laparoscopic surgery.
MeSH Terms
-
Aged
Biological Markers/blood
C-Reactive Protein/metabolism
Colectomy/*adverse effects/methods
Female
Humans
Inflammation/etiology/metabolism
Insulin-Like Growth Factor Binding Protein 3/blood
Interleukin-6/blood
Laparoscopy/adverse effects
Male
Middle Aged
Postoperative Period
Sigmoid Neoplasms/*surgery
Treatment Outcome
Vascular Endothelial Growth Factor A/blood
Figure
Reference
-
1. Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991. 1:144–150.2. Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004. 363:1187–1192.
Article3. Scheidbach H, Schneider C, Hügel O, Scheuerlein H, Bärlehner E, Konradt J, et al. Oncological quality and preliminary long-term results in laparoscopic colorectal surgery. Surg Endosc. 2003. 17:903–910.
Article4. Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002. 359:2224–2229.
Article5. Yun SS, Hwang DW, Kim SW, Park SH, Park SJ, Lee DS, et al. Better treatment strategies for patients with acute cholecystitis and American Society of Anesthesiologists classification 3 or greater. Yonsei Med J. 2010. 51:540–545.
Article6. Kwok SP, Lau WY, Carey PD, Kelly SB, Leung KL, Li AK. Prospective evaluation of laparoscopic-assisted large bowel excision for cancer. Ann Surg. 1996. 223:170–176.
Article7. Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. 2006. 93:921–928.
Article8. Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorectal Dis. 2006. 8:375–388.
Article9. Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg. 2000. 231:506–511.
Article10. Tang CL, Eu KW, Tai BC, Soh JG, MacHin D, Seow-Choen F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg. 2001. 88:801–807.
Article11. Stage JG, Schulze S, Møller P, Overgaard H, Andersen M, Rebsdorf-Pedersen VB, et al. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg. 1997. 84:391–396.
Article12. Fukushima R, Kawamura YJ, Saito H, Saito Y, Hashiguchi Y, Sawada T, et al. Interleukin-6 and stress hormone responses after uncomplicated gasless laparoscopic-assisted and open sigmoid colectomy. Dis Colon Rectum. 1996. 39:S29–S34.
Article13. Dunker MS, Ten Hove T, Bemelman WA, Slors JF, Gouma DJ, Van Deventer SJ. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral blood mononuclear cells in patients after laparoscopic vs. conventional bowel resection: a randomized study. Dis Colon Rectum. 2003. 46:1238–1244.
Article14. Delgado S, Lacy AM, Filella X, Castells A, García-Valdecasas JC, Pique JM. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum. 2001. 44:638–646.
Article15. Vittimberga FJ Jr, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998. 227:326–334.
Article16. Belizon A, Balik E, Feingold DL, Bessler M, Arnell TD, Forde KA, et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: open more so than minimally invasive methods. Ann Surg. 2006. 244:792–798.
Article17. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.
Article18. Belizon A, Balik E, Horst P, Feingold D, Arnell T, Azarani T, et al. Persistent elevation of plasma vascular endothelial growth factor levels during the first month after minimally invasive colorectal resection. Surg Endosc. 2008. 22:287–297.
Article19. Kirman I, Jain S, Cekic V, Belizon A, Balik E, Sylla P, et al. Altered plasma matrix metalloproteinase-9/tissue inhibitor of matrix [corrected] metalloproteinase-1 concentration during the early postoperative period in patients with colorectal cancer. Surg Endosc. 2006. 20:482–486.
Article20. Kirman I, Cekic V, Poltaratskaia N, Asi Z, Bessler M, Huang EH, et al. Plasma from patients undergoing major open surgery stimulates in vitro tumor growth: lower insulin-like growth factor binding protein 3 levels may, in part, account for this change. Surgery. 2002. 132:186–192.
Article21. Kirman I, Cekic V, Poltoratskaia N, Sylla P, Jain S, Forde KA, et al. Open surgery induces a dramatic decrease in circulating intact IGFBP-3 in patients with colorectal cancer not seen with laparoscopic surgery. Surg Endosc. 2005. 19:55–59.
Article22. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007. 13:2825–2830.
Article23. Kirman I, Poltaratskaia N, Cekic V, Forde KA, Ansari P, Boulay C, et al. Depletion of circulating insulin-like growth factor binding protein 3 after open surgery is associated with high interleukin-6 levels. Dis Colon Rectum. 2004. 47:911–917.
Article24. Maruszynski M, Pojda Z. Interleukin 6 (IL-6) levels in the monitoring of surgical trauma. A comparison of serum IL-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Surg Endosc. 1995. 9:882–885.25. Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, et al. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992. 111:201–209.26. Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992. 79:757–760.
Article27. Jung YD, Nakano K, Liu W, Gallick GE, Ellis LM. Extracellular signal-regulated kinase activation is required for up-regulation of vascular endothelial growth factor by serum starvation in human colon carcinoma cells. Cancer Res. 1999. 59:4804–4807.28. Gerber A, Wille A, Welte T, Ansorge S, Buhling F. Interleukin-6 and transforming growth factor-beta 1 control expression of cathepsins B and L in human lung epithelial cells. J Interferon Cytokine Res. 2001. 21:11–19.
Article29. Solís-Herruzo JA, Rippe RA, Schrum LW, de La Torre P, García I, Jeffrey JJ, et al. Interleukin-6 increases rat metalloproteinase-13 gene expression through stimulation of activator protein 1 transcription factor in cultured fibroblasts. J Biol Chem. 1999. 274:30919–30926.
Article30. Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996. 97:244–249.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laparoscopic Management of Sigmoid Volvulus for Which Endoscopic Reduction had Failed
- Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy
- Inflammatory Myofibroblastic Tumor of the Urinary Bladder Managed by Laparoscopic Partial Cystectomy
- Change of Post-anesthetic Recovery Time after Colorectal Surgery with N-acetyl-cysteine Infusion
- Granulocyte Colony Stimulating Factor Attenuates Hyperoxia-Induced Lung Injury by Down-Modulating Inflammatory Responses in Neonatal Rats