Korean J Gastroenterol.
2010 Nov;56(5):324-328. 10.4166/kjg.2010.56.5.324.
A Case of Psoriasis Induced by Infliximab Treatment for Crohn's Disease
- Affiliations
-
- 1Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea. n-hkim@paik.ac.kr
- 2Department of Dermatology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- KMID: 1718375
- DOI: http://doi.org/10.4166/kjg.2010.56.5.324
Abstract
- Infliximab, the monoclonal antibody to tumor necrosis factor, is indicated for refractory luminal and fistulizing Crohn's disease and rheumatoid arthritis. Infliximab treatment has adverse events including infusion reactions, opportunistic infections, and the potential for the event such as reactivation of latent tuberculosis. Cutaneous adverse reactions of TNF-alpha agents include skin rash, urticaria, pruritus, lupus-like eruption, and injection site reactions. Most of all, psoriasis or psoriasiform dermatitis induced by infliximab treatment for Crohn's disease is rarely reported in Korea. We report a case of psoriasis induced by infliximab treatment for Crohn's disease with a review of world literature.
Keyword
MeSH Terms
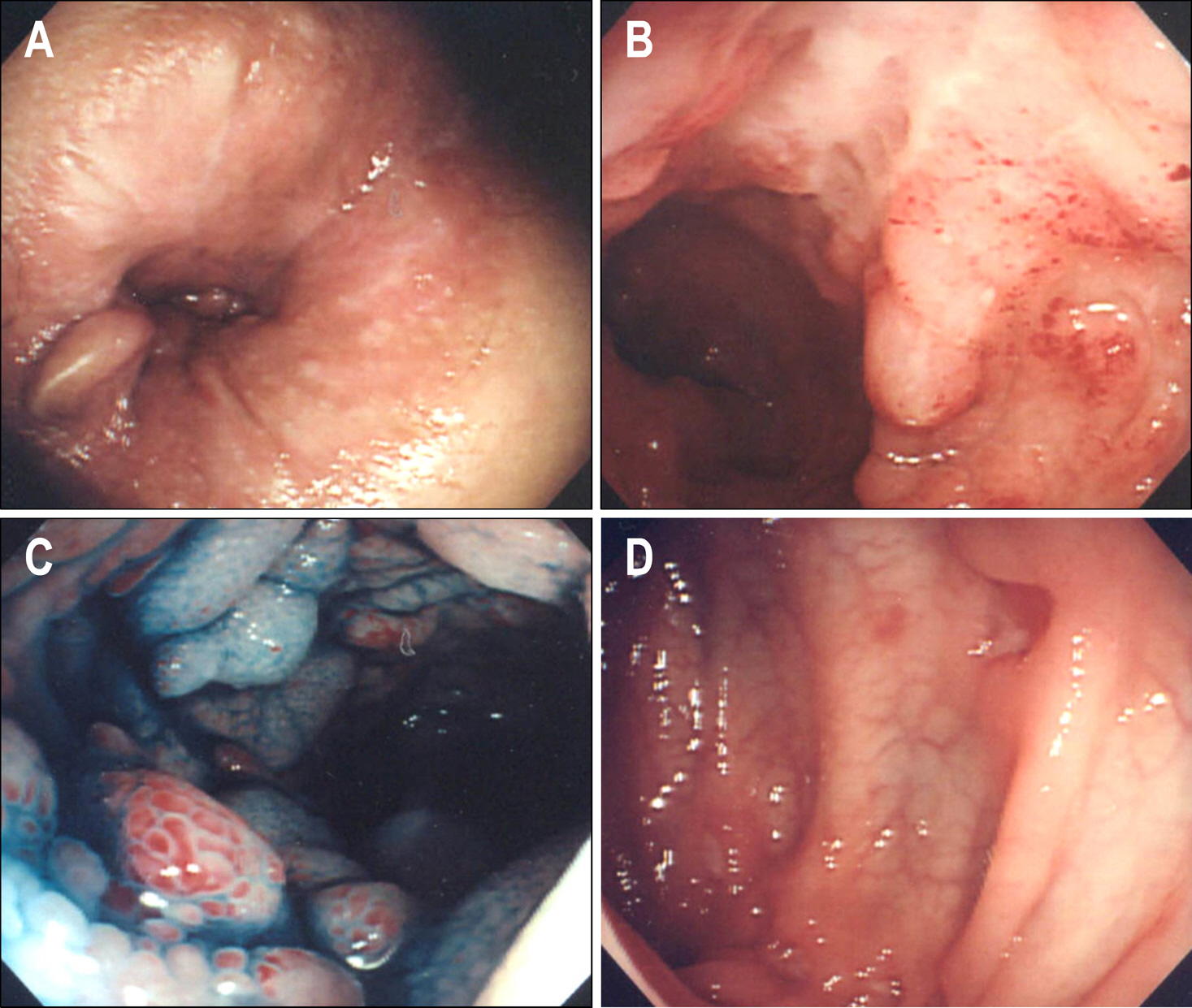
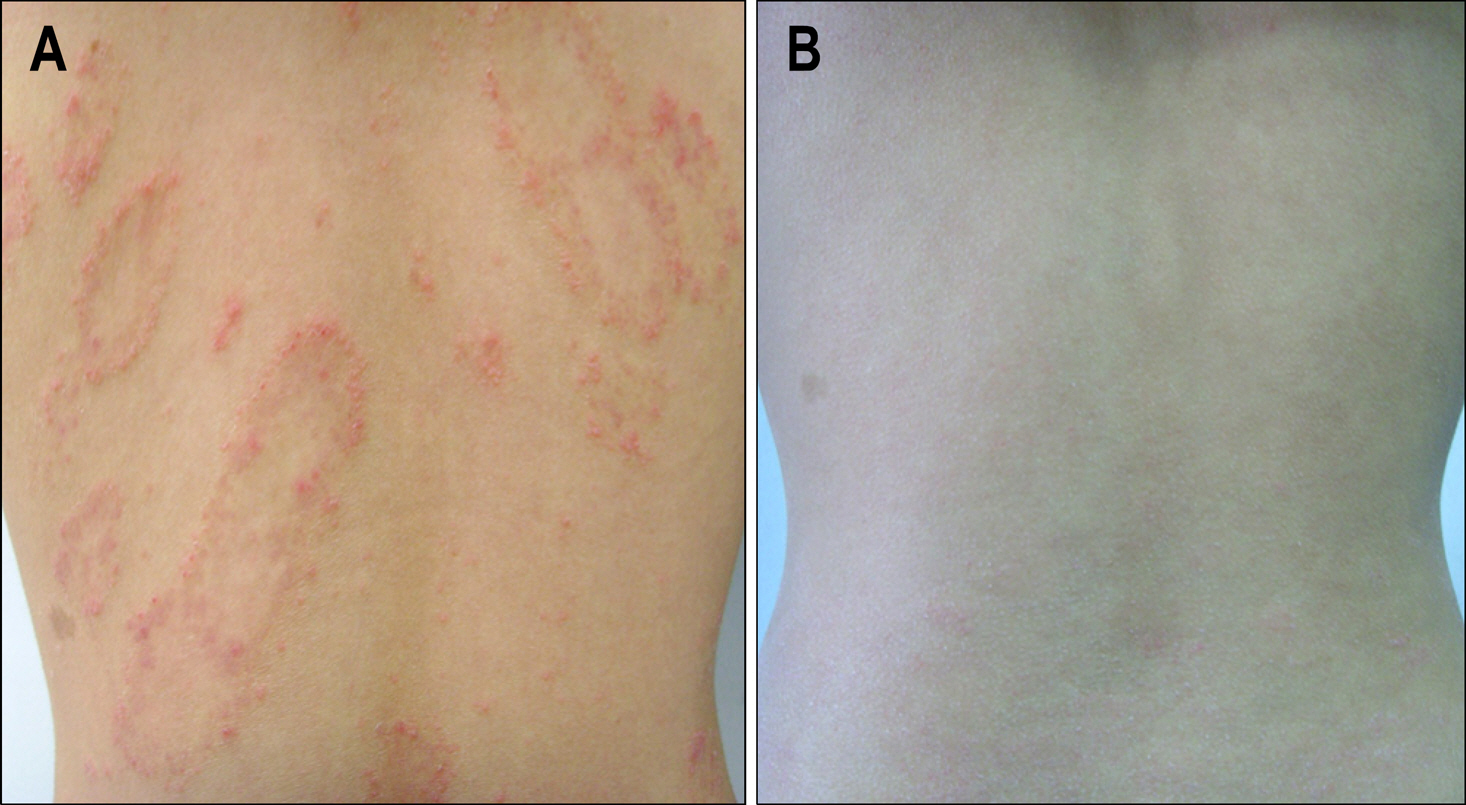
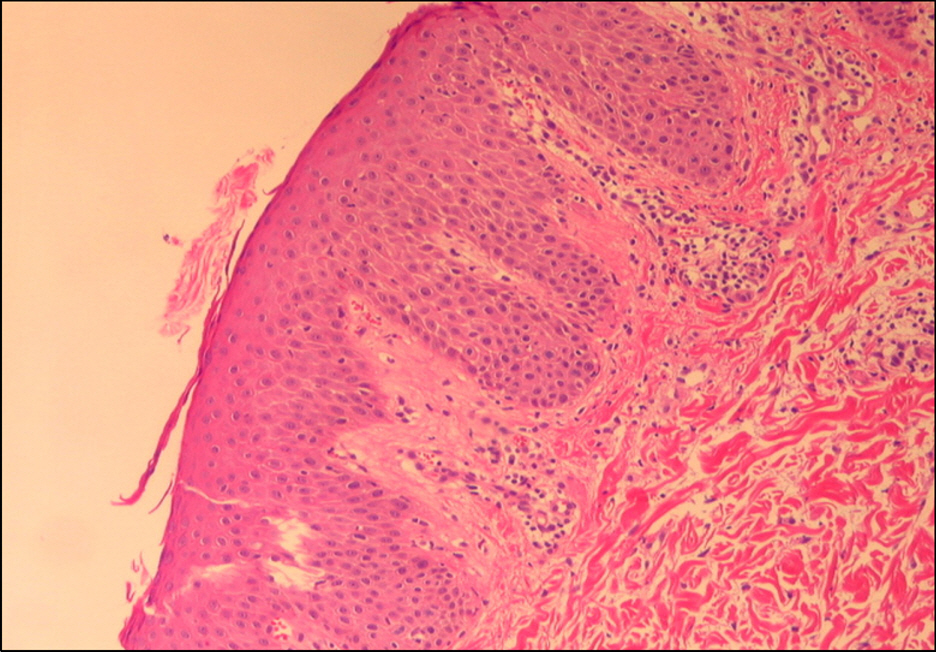
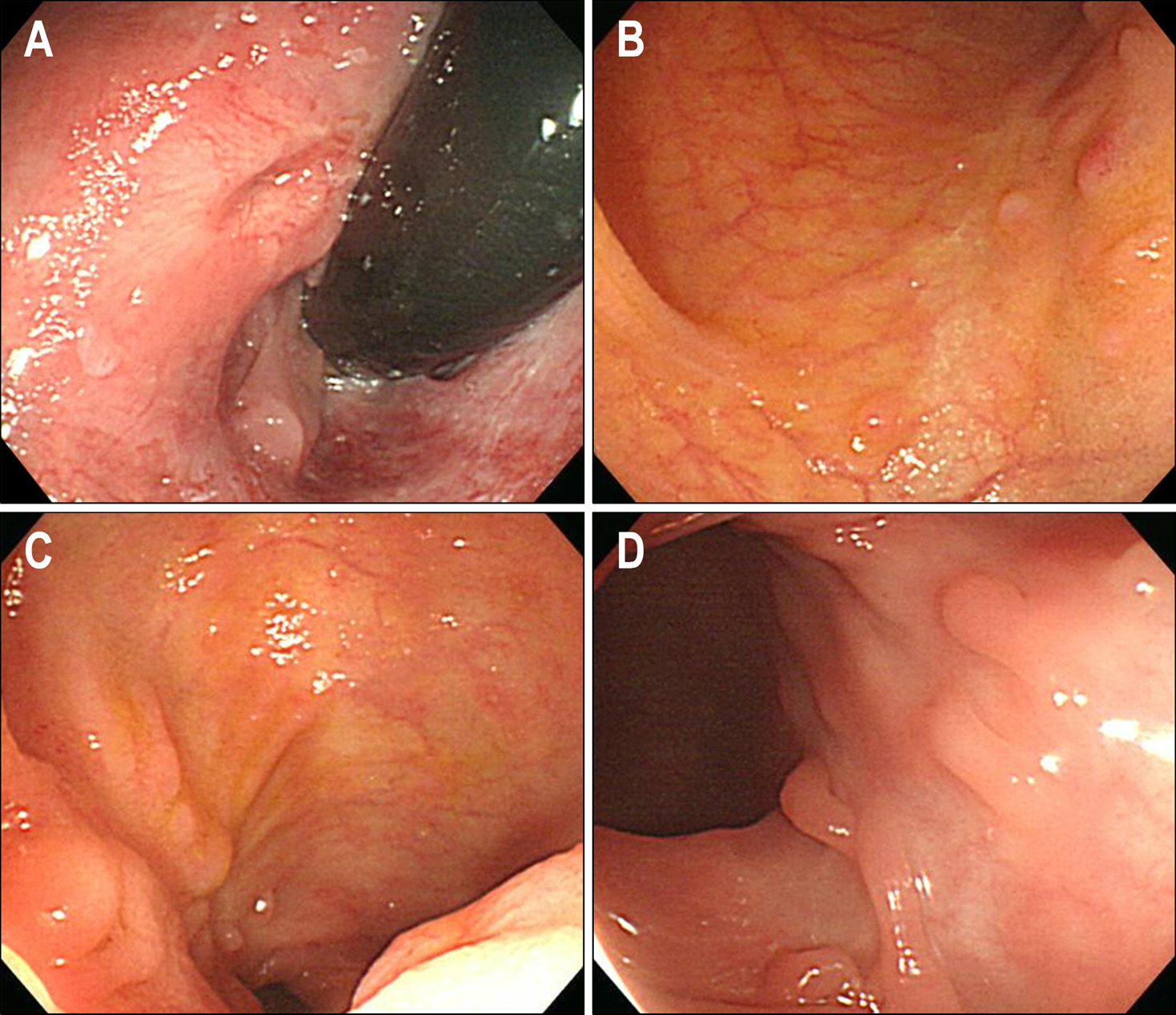
Figure
Cited by 2 articles
-
Efficacy of Early Infliximab Treatment for Pediatric Crohn's Disease: A Three-year Follow-up
Yun Seok Lee, Sang Hun Baek, Mi Jin Kim, Yoo Min Lee, Yoon Lee, Yon Ho Choe
Pediatr Gastroenterol Hepatol Nutr. 2012;15(4):243-249. doi: 10.5223/pghn.2012.15.4.243.A Case of Infliximab-induced Psoriasis in Treatment of Ankylosing Spondylitis
Min Jeong Kim, Ji Hyun Lee, Ho Joon Im, Hyun Jung Yeo, Hong Jik Lee, Ki Sup Byun
J Rheum Dis. 2014;21(5):274-277. doi: 10.4078/jrd.2014.21.5.274.
Reference
-
1. Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn's disease: a user's guide for clinicians. Am J Gastroenterol. 2002; 97:2962–2972.
Article2. Rutgeerts P, Van Assche G, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease–seven years on. Aliment Pharmacol Ther. 2006; 23:451–463.3. Fidder H, Schnitzler F, Ferrante M, et al. Longterm safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009; 58:501–508.
Article4. Wollina U, Hansel G, Koch A, Schö nlebe J, Köstler E, Haroske G. Tumor necrosis factoralpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008; 9:1–14.5. de Gannes GC, Ghoreishi M, Pope J, et al. Psoriasis and pustular dermatitis triggered by TNF-alpha inhibitors in patients with rheumatologic conditions. Arch Dermatol. 2007; 143:223–231.
Article6. Takahashi H, Hashimoto Y, Ishida-Yamamoto A, Ashida T, Kohgo Y, Iizuka H. Psoriasiform and pustular eruption induced by infliximab. J Dermatol. 2007; 34:468–472.
Article7. Severs GA, Lawlor TH, Purcell SM, Adler DJ, Thompson R. Cutaneous adverse reaction to infliximab: report of psoriasis developing in 3 patients. Cutis. 2007; 80:231–237.8. Umeno J, Matsumoto T, Jo Y, Ichikawa M, Urabe K, Iida M. Psoriasis during anti-tumor necrosis factoralpha therapy for Crohn's disease. Inflamm Bowel Dis. 2007; 13:1188–1189.
Article9. Peramiquel L, Puig L, Dalmau J, Ricart E, Roe E, Alomar A. Onset of flexural psoriasis during infliximab treatment for Crohn's disease. Clin Exp Dermatol. 2005; 30:713–714.
Article10. Angelucci E, Cocco A, Viscido A, Vernia P, Caprilli R. Another paradox in Crohn's disease: new onset of psoriasis in a patient receiving tumor necrosis factoralpha antagonist. Inflamm Bowel Dis. 2007; 13:1059–1061.11. Cohen JD, Bournerias I, Buffard V, et al. Psoriasis induced by tumor necrosis factoralpha antagonist therapy: a case series. J Rheumatol. 2007; 34:380–385.12. Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005; 52:2513–2518.
Article13. Park BC, Lim HJ, Kim BS, Lee WJ, Kim DW, Lee SJ. Repeated paradoxical aggravation of preexisting psoriasis during infliximab treatment for Crohn's disease. Ann Dermatol. 2009; 21:60–62.
Article14. Choi YJ, Kim DS, Park JM, Oh SH, Park YK, Lee JH. A case of psoriasiform eruption triggered by tumor necrosis factoralpha antagonist therapy. Korean J Dermatol. 2008; 46:721–723.15. Choi KD, Song HJ, Kim JS, Jung HC, Song IS. Efficacy and safety of treatment with Infliximab in Crohn's disease-the experience of single center in Korea. Korean J Gastroenterol. 2005; 46:48–55.16. Borrás-Blasco J, Navarro-Ruiz A, Borrás C, Casterá E. Adverse cutaneous reactions induced by TNF-alpha antagonist therapy. South Med J. 2009; 102:1133–1140.17. Mease P. TNFalpha therapy in psoriasis arthritis and psoriasis. Ann Rheum Dis. 2004; 63:755–758.18. Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn's disease. J Am Acad Dermatol. 2003; 48:805–821.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Infliximab-induced Psoriasis in Treatment of Ankylosing Spondylitis
- Repeated Paradoxical Aggravation of Preexisting Psoriasis during Infliximab Treatment for Crohn's Disease
- Development of Renal Disorder in a Patient Receiving Infliximab (Remicade(R)) for Psoriasis
- A Case of Pustular Psoriasis Developed during Infliximab Treatment for Crohn's Disease
- Infliximab: Effective Therapy for Pustular Psoriasis