Yonsei Med J.
2005 Aug;46(4):526-531. 10.3349/ymj.2005.46.4.526.
A Phase II Study of Capecitabine Combined with Gemcitabine in Patients with Advanced Gallbladder Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Yongdong Severance Hospital Pancreatobiliary Clinic, Yonsei University College of Medicine, Seoul, Korea. chojy@yumc.yonsei.ac.kr
- 2Department of Diagnostic Radiology, Yongdong Severance Hospital Pancreatobiliary Clinic, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Yongdong Severance Hospital Pancreatobiliary Clinic, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1716523
- DOI: http://doi.org/10.3349/ymj.2005.46.4.526
Abstract
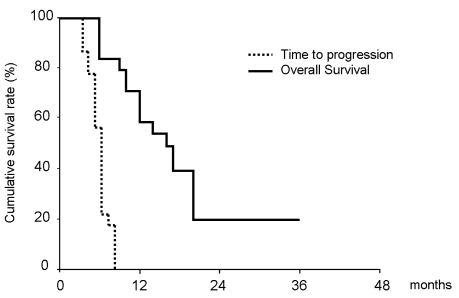
- Capecitabine and gemcitabine are used in the treatment of a variety of solid tumors including pancreatic and biliary tract carcinomas. The authors evaluated survival, response, and toxicity associated with using a combination of capecitabine and gemcitabine to treat patients with unresectable or metastatic gallbladder adenocarcinoma (GBC). Eligible patients had histologically- or cytologically-confirmed GBC, no prior systemic therapy with capecitabine or gemcitabine, Karnofsky Performance Status 70%, serum total bilirubin up to three times normal, and measurable disease. Treatment consisted of gemcitabine 1000 mg/m2 IV on Days 1 and 8 concurrent with administration of capecitabine 1000 mg/m2 PO BID on Days 1 through 14, on a 3-week cycle. Tumor response was assessed by the response evaluation criteria in solid tumors (RECIST criteria) and survival was calculated from initiation of CapGem therapy. A total of 24 patients were enrolled. Median age at the time of diagnosis was 62 years (range, 41-78 years). Fourteen patients had undergone prior surgery. Results showed that eight patients achieved partial response (33%) with an additional 10 patients achieving stable disease (42%). The overall median time to disease progression was 6.0 months (95% CI, 3.8-8.1 months) and overall survival was 16 months (95% CI, 13.8-18.3 months). The one-year survival rate was 58%. No Grade 4 toxicity was seen. Transient Grade 3 neutropenia/ thrombocytopenia and manageable nausea, hand-foot syndrome and anorexia were the most common toxicities. Our study shows that CapGem is an active and well-tolerated chemotherapy regimen in patients with advanced GBC.
Keyword
MeSH Terms
Figure
Reference
-
1. Verderame F, Mandina P, Abruzzo F, Scarpulla M, Di Leo R. Biliary tract cancer: our experience with gemcitabine treatment. Anticancer Drugs. 2000; 11:707–708. PMID: 11129732.
Article2. Murakami K, Tanimura H, Yamaue H, Mizobata S, Noguchi K, Nakamori M, et al. Clinical effect of immunochemotherapy for a patient with advanced gallbladder cancer: report of a case. Surg Today. 1998; 28:923–928. PMID: 9744401.
Article3. Hasegawa H, Ueo H, Nanbara S, Tsuji K, Mori M, Akiyoshi T. An effective pre-operative chemoimmunotherapy regimen against advanced gallbladder carcinoma: a case report. Hepatogastroenterology. 1999; 46:1639–1642. PMID: 10430311.4. Hara Y, Kawasaki T, Yabata E, Gen T, Jibiki M, Kudoh A, et al. A case of unresectable gallbladder cancer responding to combination therapy with hyperthermia and local chemotherapy. Gan To Kagaku Ryoho. 2000; 27:117–120. PMID: 10660743.5. Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003; 9:693–702. PMID: 12576437.6. Gallardo J, Fodor M, Gamargo C, Orlandi L. Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer. 1998; 83:2419–2421. PMID: 9840543.7. Castro MP. Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer. 1998; 82:639–641. PMID: 9477094.8. Gallardo JO, Rubio B, Fodor M, Orlandi L, Yanez M, Gamargo C, et al. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol. 2001; 12:1403–1436. PMID: 11762811.
Article9. Raderer M, Hejna MH, Valencak JB, Kornek GV, Weinlander GS, Bareck E, et al. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology. 1999; 56:177–180. PMID: 10202270.
Article10. Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001; 12:183–186. PMID: 11300321.
Article11. Bruckner HW, Zhou G, Haenel P. Ex vivo ATP tumor testing of gemcitabine for combination chemotherapy and biochemical modulation. Proc Am Assoc Cancer Res. 1998; 39:310.12. Poplin E, Roberts J, Tombs M, Grant S, Rubin E. Leucovorin, 5-fluorouracil, and gemcitabine: a phase I study. Invest New Drugs. 1999; 17:57–62. PMID: 10555123.13. Hidalgo M, Castellano D, Paz-Ares L, Gravalos C, Diaz-Puente M, Hitt R, et al. Phase I-II study of gemcitabine and fluorouracil as a continuous infusion in patients with pancreatic cancer. J Clin Oncol. 1999; 17:585–592. PMID: 10080603.
Article14. Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000; 45:291–297. PMID: 10755317.15. Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998; 34:1274–1281. PMID: 9849491.
Article16. Endo M, Miwa M, Eda H, Ura M, Tanimura H, Ishikawa T, et al. Induction of thymidine phosphorylase expression and enhancement of efficacy of capecitabine or 5-deoxy-5-fluoroudine by cyclophosphamide in mammary tumor models. Int J Cancer. 1999; 83:127–134. PMID: 10449619.17. Twelves C, Glynne-Jones R, Cassidy J, Schuller J, Goggin T, Roos B, et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res. 1999; 5:1696–1702. PMID: 10430071.18. Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized Phase III study. J Clin Oncol. 2001; 19:2282–2292. PMID: 11304782.
Article19. Rudi J. Chemotherapy with gemcitabine in patients with gall-bladder carcinoma. Ann Oncol. 2002; 13:807. PMID: 12075753.
Article20. Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004; 101:578–586. PMID: 15274071.
Article21. Knox JJ, Hedley D, Oza A, Siu E, Chen R, Feld M, et al. Phase II trial of gemcitabine plus capecitabine (GemCap) in patients with advanced or metastatic adenocarcinoma of the biliary tract. Proc Am Soc Clin Oncol. 2003; 22:317.22. Carraro S, Servienio PJ, Bruno MF, Castillo Odena MDS, Roca E, Jovtis S, et al. Gemcitabine and cisplatin in locally advanced or metastatic gallbladder and bile duct adenocarcinomas. Proc Am Soc Clin Oncol. 2001; 20:A2333.23. Gebbia V, Giuliani F, Maiello E, Colucci G, Verderame F, Borsellino N, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol. 2001; 19:4089–4091. PMID: 11600613.24. Kuhn R, Ridwelski K, Eichelmann K, Rudolph S, Fahlke J, Ridwelski K. Outpatient combination chemotherapy with gemcitabine and docetaxel in patients (Pts) with cancer of the biliary system. Proc Am Soc Clin Oncol. 2001; 20:A2272.25. Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, et al. Mitomycin C in combination with capecitabine or biweekly high dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004; 15:478–483. PMID: 14998852.26. Scheithauer W. Review of gemcitabine in biliary tract carcinoma. Semin Oncol. 2002; 29:40–45. PMID: 12577232.
Article27. Murad AM, Guimara RC, Aragao BC, Rodrigues VH, Scalabrini-Neto AO, Padua CA, et al. Phase II trial of the use of gemcitabine and 5-fluorouracil in the treatment of advanced pancreatic and biliary tract cancer. Am J Clin Oncol. 2003; 26:151–154. PMID: 12714886.
Article28. Malik IA, Aziz Z, Zaidi SHM, Sethuraman G. Gemcitabine and cisplatin is a highly effective combination chemotherapy in patients with advanced cancer of the gall bladder. Am J Clin Oncol. 2003; 26:174–177. PMID: 12714891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Biliary Tract Cancer
- A Phase II Trial of Gemcitabine plus Capecitabine for Patients with Advanced Pancreatic Cancer
- Gemcitabine-based Chemotherapy for Gallbladder Cancer
- Gemcitabine Combined with Capecitabine Compared to Gemcitabine with or without Erlotinib as First-Line Chemotherapy in Patients with Advanced Pancreatic Cancer
- A Phase II Study of Combination Chemotherapy with Gemcitabine, 5-fluorouracil, and Cisplatin for Advanced Pancreatic Cancer