Cancer Res Treat.
2012 Jun;44(2):127-132.
A Phase II Trial of Gemcitabine plus Capecitabine for Patients with Advanced Pancreatic Cancer
- Affiliations
-
- 1Division of Oncology-Hematology, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea. kjs6651@kumc.or.kr
Abstract
- PURPOSE
The purpose of this study was to determine the efficacy and safety of treatment using gemcitabine and capecitabine for patients with advanced pancreatic cancer.
MATERIALS AND METHODS
Patients with advanced unresectable pancreatic adenocarcinoma were enrolled in the study. Inclusion criteria included no prior systemic chemotherapy or radiation therapy, at least one radiographically documented and measurable tumor lesion, and adequate patient organ functions. The patients received 1,000 mg/m2 gemcitabine intravenously on days 1, 8 and 15, and 830 mg/m2 of oral capecitabine twice a day on days 1-21 of a 28-day cycle.
RESULTS
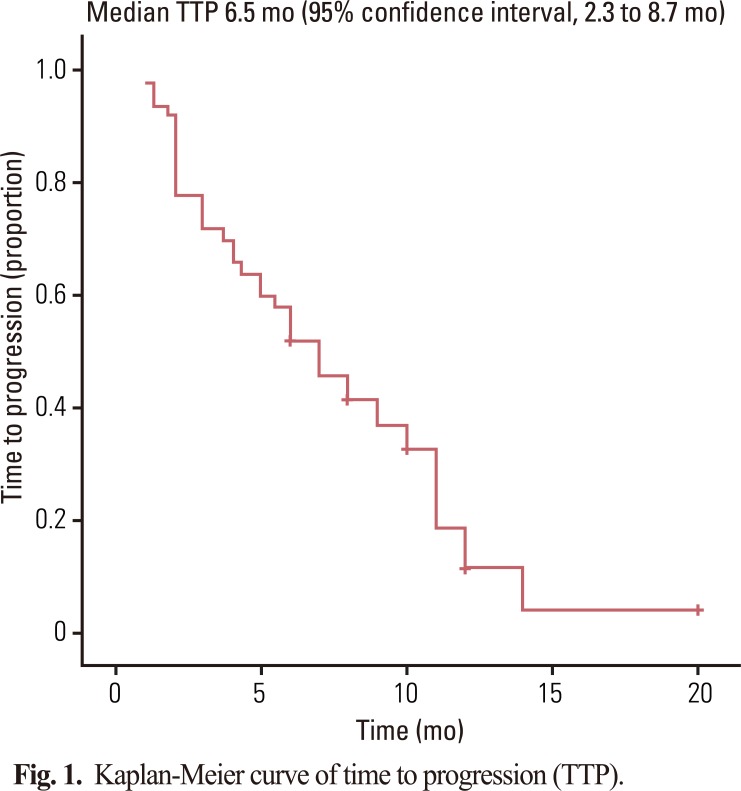
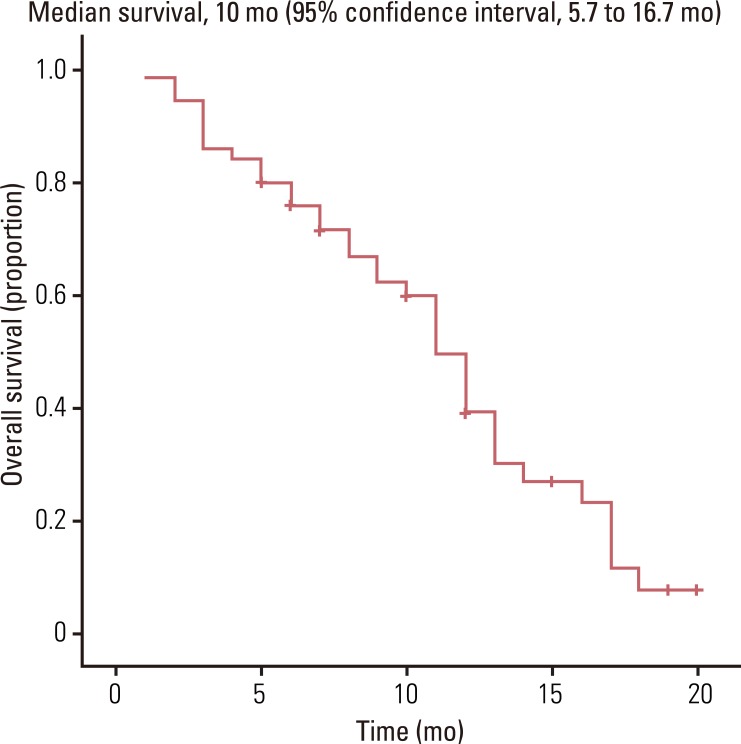
Fifty patients with a median age of 53 years (range, 39 to 76 years) were enrolled in the study. The median follow-up was 10.0 months. The objective response rate of the 50 patients was 48.0% (95% CI, 22.5 to 57.1%). The median time to progression and overall survival were 6.5 months (95% CI, 2.3 to 8.7 months) and 10.0 months (95% CI, 5.7 to 16.7 months), respectively. Grade 3-4 toxicities associated with chemotherapy included neutropenia (22%), anemia (8%), thrombocytopenia (6%), and hand-foot syndrome (10%).
CONCLUSION
Combination chemotherapy using gemcitabine and capecitabine was well tolerated and demonstrated promising efficacy in the treatment of advanced pancreatic cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Koorstra JB, Hustinx SR, Offerhaus GJ, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008; 8:110–125. PMID: 18382097.
Article2. Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer. 1999; 85:1261–1268. PMID: 10189130.3. Cullinan S, Moertel CG, Wieand HS, Schutt AJ, Krook JE, Foley JF, et al. A phase III trial on the therapy of advanced pancreatic carcinoma. Evaluations of the Mallinson regimen and combined 5-fluorouracil, doxorubicin, and cisplatin. Cancer. 1990; 65:2207–2212. PMID: 2189551.
Article4. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–2413. PMID: 9196156.
Article5. Barhoumi M, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Locally advanced unresectable pancreatic cancer: induction chemoradiotherapy followed by maintenance gemcitabine versus gemcitabine alone: definitive results of the 2000-2001 FFCD/SFRO phase III trial. Cancer Radiother. 2011; 15:182–191. PMID: 21315644.6. Oettle H, Arning M, Pelzer U, Arnold D, Stroszczynski C, Langrehr J, et al. A phase II trial of gemcitabine in combination with 5-fluorouracil (24-hour) and folinic acid in patients with chemonaive advanced pancreatic cancer. Ann Oncol. 2000; 11:1267–1272. PMID: 11106115.
Article7. Matano E, Tagliaferri P, Libroia A, Damiano V, Fabbrocini A, De Lorenzo S, et al. Gemcitabine combined with continuous infusion 5-fluorouracil in advanced and symptomatic pancreatic cancer: a clinical benefit-oriented phase II study. Br J Cancer. 2000; 82:1772–1775. PMID: 10839289.
Article8. Cartwright TH, Cohn A, Varkey JA, Chen YM, Szatrowski TP, Cox JV, et al. Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol. 2002; 20:160–164. PMID: 11773165.
Article9. Stathopoulos GP, Syrigos K, Polyzos A, Fountzilas G, Rigatos SK, Ziras N, et al. Front-line treatment of inoperable or metastatic pancreatic cancer with gemcitabine and capecitabine: an intergroup, multicenter, phase II study. Ann Oncol. 2004; 15:224–229. PMID: 14760113.
Article10. Hess V, Salzberg M, Borner M, Morant R, Roth AD, Ludwig C, et al. Combining capecitabine and gemcitabine in patients with advanced pancreatic carcinoma: a phase I/II trial. J Clin Oncol. 2003; 21:66–68. PMID: 12506172.
Article11. Scheithauer W, Schüll B, Ulrich-Pur H, Schmid K, Raderer M, Haider K, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol. 2003; 14:97–104. PMID: 12488300.
Article12. Schilsky RL, Bertucci D, Vogelzang NJ, Kindler HL, Ratain MJ. Dose-escalating study of capecitabine plus gemcitabine combination therapy in patients with advanced cancer. J Clin Oncol. 2002; 20:582–587. PMID: 11786589.
Article13. Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009; 27:5513–5518. PMID: 19858379.
Article14. Park BB, Park JO, Lee HR, Lee J, Choi DW, Choi SH, et al. A phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2007; 60:489–494. PMID: 17396266.
Article15. Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996; 73:101–105. PMID: 8554969.
Article16. Rocha Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004; 22:3776–3783. PMID: 15365074.
Article17. Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005; 23:3509–3516. PMID: 15908661.
Article18. Abou-Alfa GK, Letourneau R, Harker G, Modiano M, Hurwitz H, Tchekmedyian NS, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol. 2006; 24:4441–4447. PMID: 16983112.
Article19. Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005; 16:1639–1645. PMID: 16087696.
Article20. Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004; 22:1430–1438. PMID: 15084616.
Article21. Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007; 25:2212–2217. PMID: 17538165.
Article22. Harsha HC, Jimeno A, Molina H, Mihalas AB, Goggins MG, Hruban RH, et al. Activated epidermal growth factor receptor as a novel target in pancreatic cancer therapy. J Proteome Res. 2008; 7:4651–4658. PMID: 18821783.
Article23. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–1966. PMID: 17452677.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Pancreatic Cancer
- Chemotherapy for Biliary Tract Cancer
- A Phase II Study of Combination Chemotherapy with Gemcitabine, 5-fluorouracil, and Cisplatin for Advanced Pancreatic Cancer
- Gemcitabine Combined with Capecitabine Compared to Gemcitabine with or without Erlotinib as First-Line Chemotherapy in Patients with Advanced Pancreatic Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma