J Korean Med Sci.
2010 May;25(5):798-803. 10.3346/jkms.2010.25.5.798.
Primary Culture of Central Neurocytoma: A Case Report
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul National University, Seoul, Korea. gknife@plaza.snu.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul National University, Seoul, Korea.
- 3Laboratory of Neuroprotection, Division of Life Pharmaceutical Sciences, Brain Disease Rescarch Institute, College of Pharmacy, Ewha Woman's University, Seoul, Korea.
- KMID: 1713970
- DOI: http://doi.org/10.3346/jkms.2010.25.5.798
Abstract
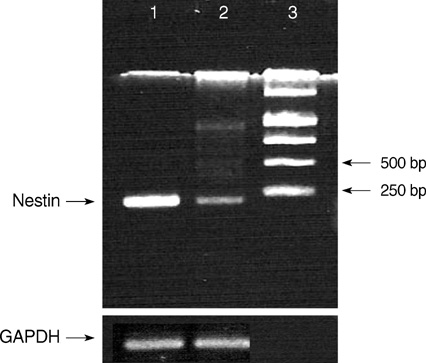
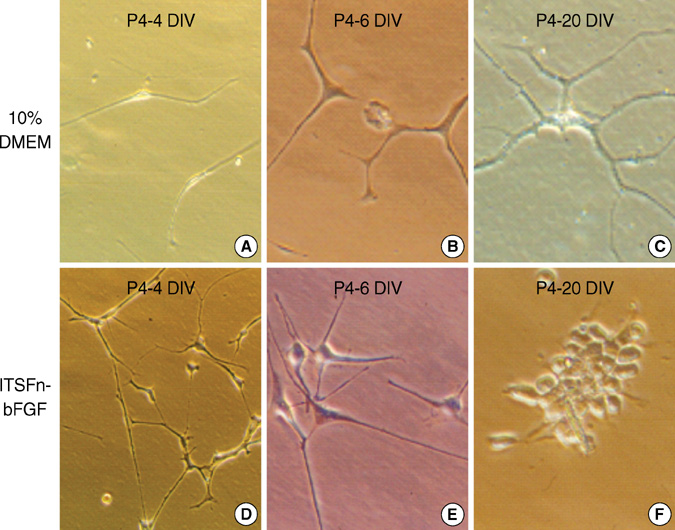
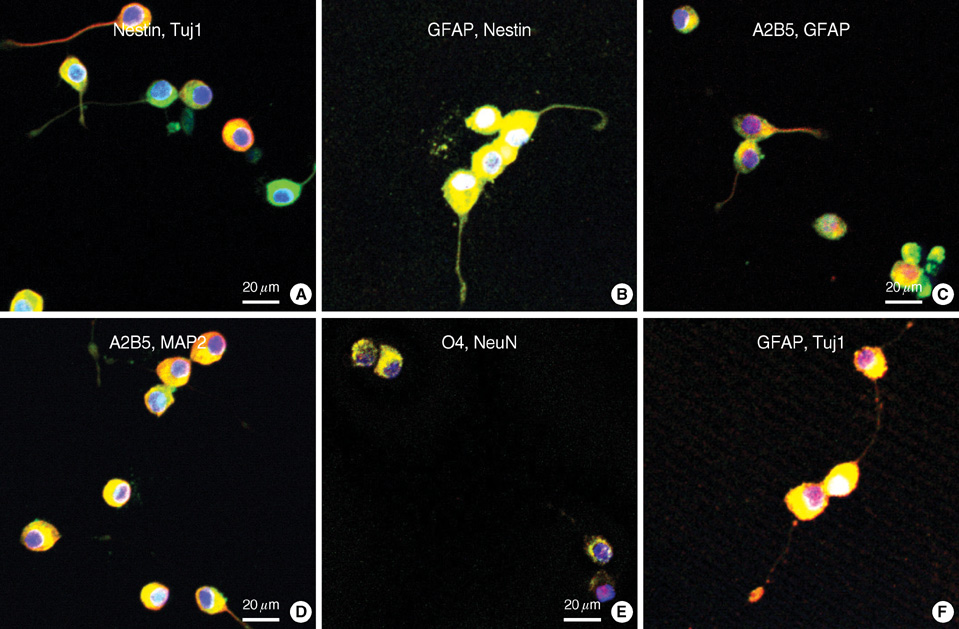
- A seventeen-year-old female patient was admitted with sudden-onset of headache and vomiting. Brain magnetic resonance imaging demonstrated a heterogeneously enhancing tumour in the left lateral ventricle. The tumour was removed and confirmed as a central neurocytoma (CN). For the residual tumour in the left lateral ventricle, gamma knife stereotactic radiosurgery was done at fifteen months after the initial surgery. Tumour recurred in the 4th ventricle at 5 yr after initial surgery. The tumour was removed and proved as a CN. In vitro primary culture was done with both tumours obtained from the left lateral ventricle and the 4th ventricle, respectively. Nestin, a neuronal stem cell marker was expressed in reverse Transcriptase-Polymerase Chain Reaction of both tumors. Both tumours showed different morphology and phenotypes of neuron and glia depending on the culture condition. When cultured in insulin, transferrin selenium and fibronectin media with basic fibroblast growth factors, tumour cells showed neuronal morphology and phenotypes. When cultured in the Dulbeco's Modified Essential Media with 20% fetal bovine serum, tumors cells showed glial morphology and phenotypes. It is suggested that CN has the characteristics of neuronal stem cells and potential to differentiate into mature neuron and glial cells depending on the environmental cue.
MeSH Terms
Figure
Reference
-
1. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001. 414:105–111.
Article2. Temple S. The development of neural stem cells. Nature. 2001. 414:112–117.
Article3. Doetsch F, Garcia_Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997. 17:5046–5061.
Article4. Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002. 22:612–613.
Article5. Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, Toga M. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982. 56:151–156.6. Von Deimling A, Kleihues P, Saremaslani P, Yasargil MG, Spoerri O, Sudhof TC, Wiestler OD. Histogenesis and differentiation potential of central neurocytomas. Lab Invest. 1991. 64:585–591.7. Ishiuchi S, Nakazato Y, Iino M, Ozawa S, Tamura M, Ohye C. In vitro neuronal and glial production and differentiation of human central neurocytoma cells. J Neurosci Res. 1998. 51:526–535.
Article8. Patt S, Schmidt H, Labrakakis C, Weydt P, Fritsch M, Cervos-Navarro J, Kettenmann H. Human central neurocytoma cells show neuronal physiological properties in vitro. Acta Neuropathol. 1996. 91:209–214.
Article9. Tsuchida T, Yamada A, Yoshimura K, Kawamoto K. Ultrastructural characterization of central neurocytomas using collagen gel culture. Ultrastruct Pathol. 1998. 22:233–238.
Article10. Valdueza JM, Westphal M, Vortmeyer A, Muller D, Padberg B, Hermann HD. Central neurocytoma: clinical, immunohistologic, and biologic findings of a human neuroglial progenitor tumor. Surg Neurol. 1996. 45:49–56.
Article11. Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992. 66:303–313.12. Raff MC, Miller RH, Noble MA. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983. 303:390–396.
Article13. Tardy M, Fages C, Riol H, LePrince G, Rataboul P, Charriere-Bertrand C, Nunez J. Developmental expression of the glial fibrillary acidic protein mRNA in the central nervous system and in cultured astrocytes. J Neurochem. 1989. 52:162–167.
Article14. Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting revealed a neuronal lineage. Development. 2000. 127:5253–5263.15. Satoh J, Kim SU. Ganglioside markers GD3, GD2, and A2B5 in fetal human neurons and glial cells in culture. Dev Neurosci. 1995. 17:137–148.
Article16. Gensburger C, Capo M, Deloulme JC, Sensenbrenner M. Influence of basic fibroblast growth factor on the development of cholinoreceptive neurons from fetal rat cerebrum in culture. Dev Neurosci. 1992. 14:278–281.17. Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993. 10:255–265.
Article18. Kato T, Heike T, Okawa K, Haruyama M, Shiraishi K, Yoshimoto M, Nagato M, Shibata M, Kumada T, Yamanaka Y, Hattori H, Nakahata T. A neurosphere-derived factor, cystatin C, supports differentiation of ES cells into neural stem cells. Proc Natl Acad Sci USA. 2006. 103:6019–6024.
Article19. Kim BJ, Kim SS, Kim YI, Paek SH, Lee YD, Suh-Kim H. Forskolin promotes astroglial differentiation of human central neurocytoma cells. Exp Mol Med. 2004. 36:52–56.
Article20. Sim FJ, Keyoung HM, Goldman JE, Kim DK, Jung HW, Roy NS, Goldman SA. Neurocytoma is a tumor of adult neuronal progenitor cells. J Neurosci. 2006. 26:12544–12555.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Cell Culture with Immunocytochemistry and Electron Microscopy to the Diagnosis of Central Neurocytoma
- Cerebral Central Neurocytoma with High Proliferative Index: Case Report
- Central Neurocytoma Presenting as Symptomatic Cataplexy
- Culture Characteristics of Oligodendroglioma and Central Neurocytoma
- Central Neurocytoma Originated from Atrium with Malignant Trans formation: A Case Report