J Korean Med Sci.
2010 May;25(5):781-784. 10.3346/jkms.2010.25.5.781.
Hematopoietic Stem Cell Transplantation after Posttransplant Lymphoproliferative Disorder
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. kim_dajung@hanmail.net
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1713966
- DOI: http://doi.org/10.3346/jkms.2010.25.5.781
Abstract
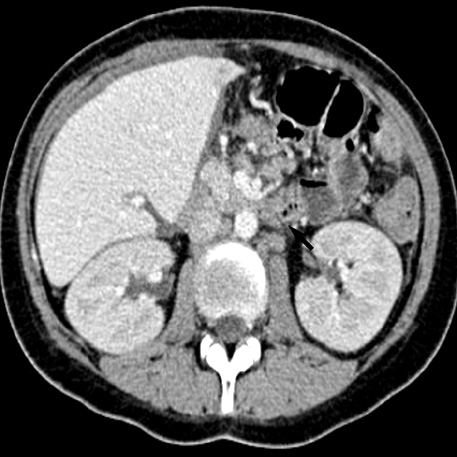
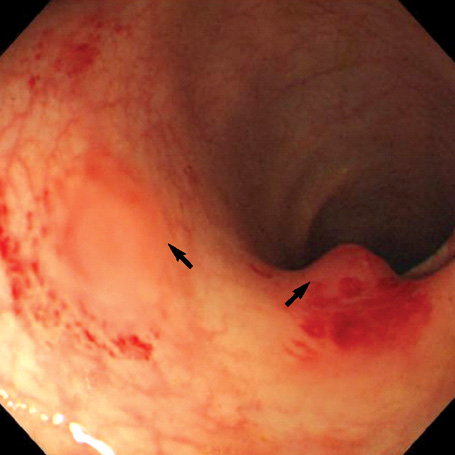
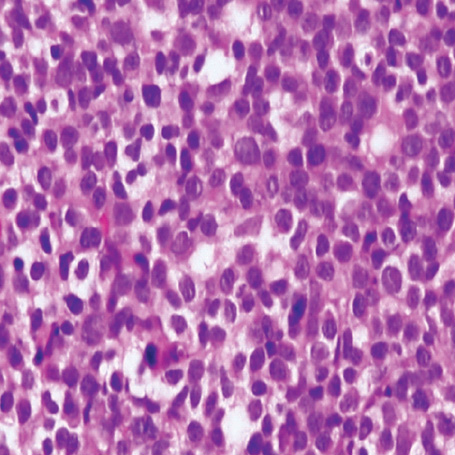
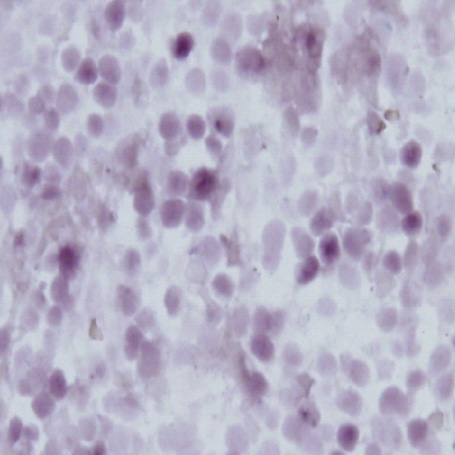
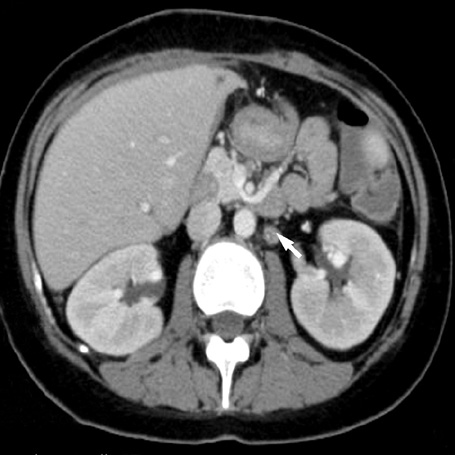
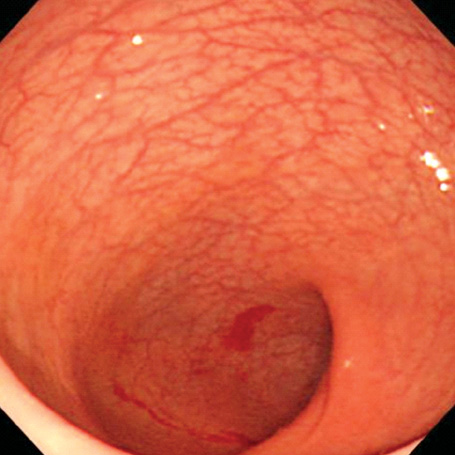
- A 16-yr-old girl received liver transplantation for fulminant hepatitis. Aplastic anemia developed, and she received hematopoietic stem cell transplantation (HSCT). Eleven months after liver transplantation, abdominal lymph node enlargement and colon ulcers were observed, and colon biopsy showed posttransplant lymphoproliferative disorder (PTLD). Immunosuppression reduction was attempted, but it produced no therapeutic effect. Fourteen months after liver transplantation, she received a second HSCT due to engraftment failure, and PTLD resolved completely. The second HSCT can serve as cellular therapy for PTLD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Serongative Acute Hepatic Failure-associated Aplastic Anemia in Pediatric Liver Transplantation
Chul Han Eon, Nam-Joon Yi, Geun Hong, Su Park Min, Rok Choi Young, Heyoung Kim, Woong Lee Kwang, Ho Kim In, Jun Kim Yoon, Sung Ko Jae, Duk Park Kyung, Jong Lee Hoan, Hwa Choi Eun, Kee Seo Jeong, Kyoung-Bun Lee, Suk Suh Kyung
J Korean Soc Transplant. 2011;25(4):276-281. doi: 10.4285/jkstn.2011.25.4.276.
Reference
-
1. Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003. 89:1221–1227.
Article2. Curtis RE, Travis LB, Rowlings PA, Socié G, Kingma DW, Banks PM, Jaffe ES, Sale GE, Horowitz MM, Witherspoon RP, Shriner DA, Weisdorf DJ, Kolb HJ, Sullivan KM, Sobocinski KA, Gale RP, Hoover RN, Fraumeni JF, Deeg HJ. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999. 94:2208–2216.3. Walker RC, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, McGregor CG, Paya CV. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995. 20:1346–1353.
Article4. Cox KL, Lawrence-Miyasaki LS, Garcia-Kennedy R, Lennette ET, Martinez OM, Krams SM, Berquist WE, So SK, Esquivel CO. An increased incidence of Epstein-Barr virus infection and lymphoproliferative disorder in young children on FK506 after liver transplantation. Transplantation. 1995. 59:524–529.5. Beveridge T, Krupp P, McKibbin C. Lymphomas and lymphoproliferative lesions developing under cyclosporin therapy. Lancet. 1984. 1:788.
Article6. Trofe J, Beebe TM, Buell JF, Hanaway MJ, First MR, Alloway RR, Gross TG, Woodle ES. Posttransplant malignancy. Prog Transplant. 2004. 14:193–200.
Article7. Hanto DW. Classification of Epstein-Barr virus-associated posttransplant lymphoproliferative diseases: Implications for understanding their pathogenesis and developing rational treatment strategies. Annu Rev Med. 1995. 46:381–394.8. Lucas KG, Small TN, Heller G, Dupont B, O'Reilly RJ. The development of cellular immunity to Epstein-Barr virus after allogeneic bone marrow transplantation. Blood. 1996. 87:2594–2603.
Article9. Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N, Rickinson AB, Leiner I, Pamer E, Lowenberg B, Cornelissen JJ, Gratama JW. Impaired recovery of Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003. 101:4290–4297.
Article10. Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000. 343:481–492.
Article11. American Society of Transplantation. Epstein-Barr virus and lymphoproliferative disorders after transplantation. Am J Transplant. 2004. 4:Suppl 10. 59–65.12. Tanner JE, Alfieri C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: Interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl Infect Dis. 2001. 3:60–69.
Article13. Jain AB, Marcos A, Pokharna R, Shapiro R, Fontes PA, Marsh W, Mohanka R, Fung JJ. Rituximab (chimeric anti-CD20 antibody) for posttransplant lymphoproliferative disorder after solid organ transplantation in adults: long-term experience from a single center. Transplantation. 2005. 80:1692–1698.
Article14. Milpied N, Vasseur B, Parquet N, Garnier JL, Antoine C, Quartier P, Carret AS, Bouscary D, Faye A, Bourbigot B, Reguerre Y, Stoppa AM, Bourquard P, Hurault de Ligny B, Dubief F, Mathieu-Boue A, Leblond V. Humanized anti-CD20 monoclonal antibody (Rituximab) in post-transplant B lymphoproliferative disorder: a retrospective analysis on 32 patients. Ann Oncol. 2000. 11:Suppl 1. 113–116.15. Garcia VD, Bonamigo Filho JL, Neumann J, Fogliatto L, Geiger AM, Garcia CD, Barros V, Keitel E, Bittar AE, Ferrera des Santos A, Roithmann S. Rituximab in association with rapamycin for post-transplant lymphoproliferative disease treatment. Transpl Int. 2003. 16:202–206.
Article16. Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, Young JW, Kernan NA, O'Reilly RJ. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994. 330:1185–1191.
Article17. O'Reilly RJ, Small TN, Papadopoulos E, Lucas K, Lacerda J, Koulova L. Biology and adoptive cell therapy of Epstein-Barr virus-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol Rev. 1997. 157:195–216.18. Haque T, Taylor C, Wilkie GM, Murad P, Amlot PL, Beath S, McKiernan PJ, Crawford DH. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein-Barr virus-specific cytotoxic T cells 1. Transplantation. 2001. 72:1399–1402.19. Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998. 92:1549–1555.
Article20. Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, Dombagoda D, Britton KM, Swerdlow AJ, Crawford DH. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002. 360:436–442.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Hodgkin's Lymphoma after Allogeneic Hematopoietic Cell Transplantation
- Hodgkin's Lymphoma-like Posttransplantation Lymphoproliferative Disorder after Allogeneic Hematopoietic Stem Cell Transplantation
- Clinical Characteristics and Outcomes of Posttransplant Lymphoproliferative Disorders Following Allogeneic Hematopoietic Stem Cell Transplantation in Korea
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation