J Korean Med Sci.
2008 Apr;23(2):307-314. 10.3346/jkms.2008.23.2.307.
Expression of Toll-Like Receptors in Verruca and Molluscum Contagiosum
- Affiliations
-
- 1Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea. yymmpark@hotmail.com
- 2Department of Dermatology, University of Colorado at Denver and Health Science Center, Denver, Colorado, U.S.A.
- KMID: 1713466
- DOI: http://doi.org/10.3346/jkms.2008.23.2.307
Abstract
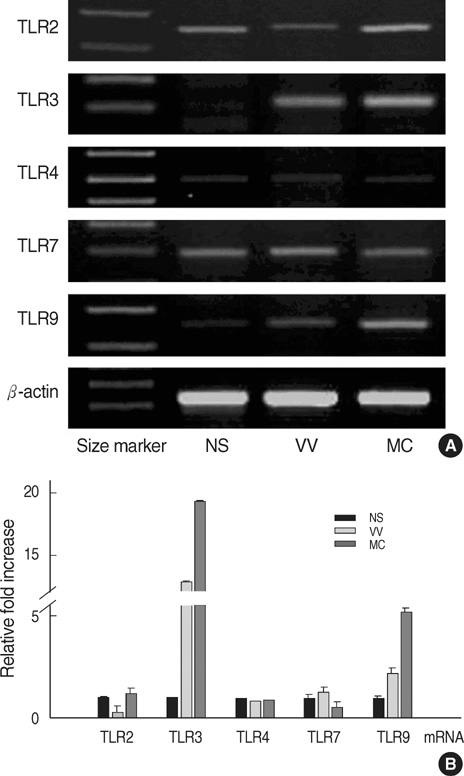
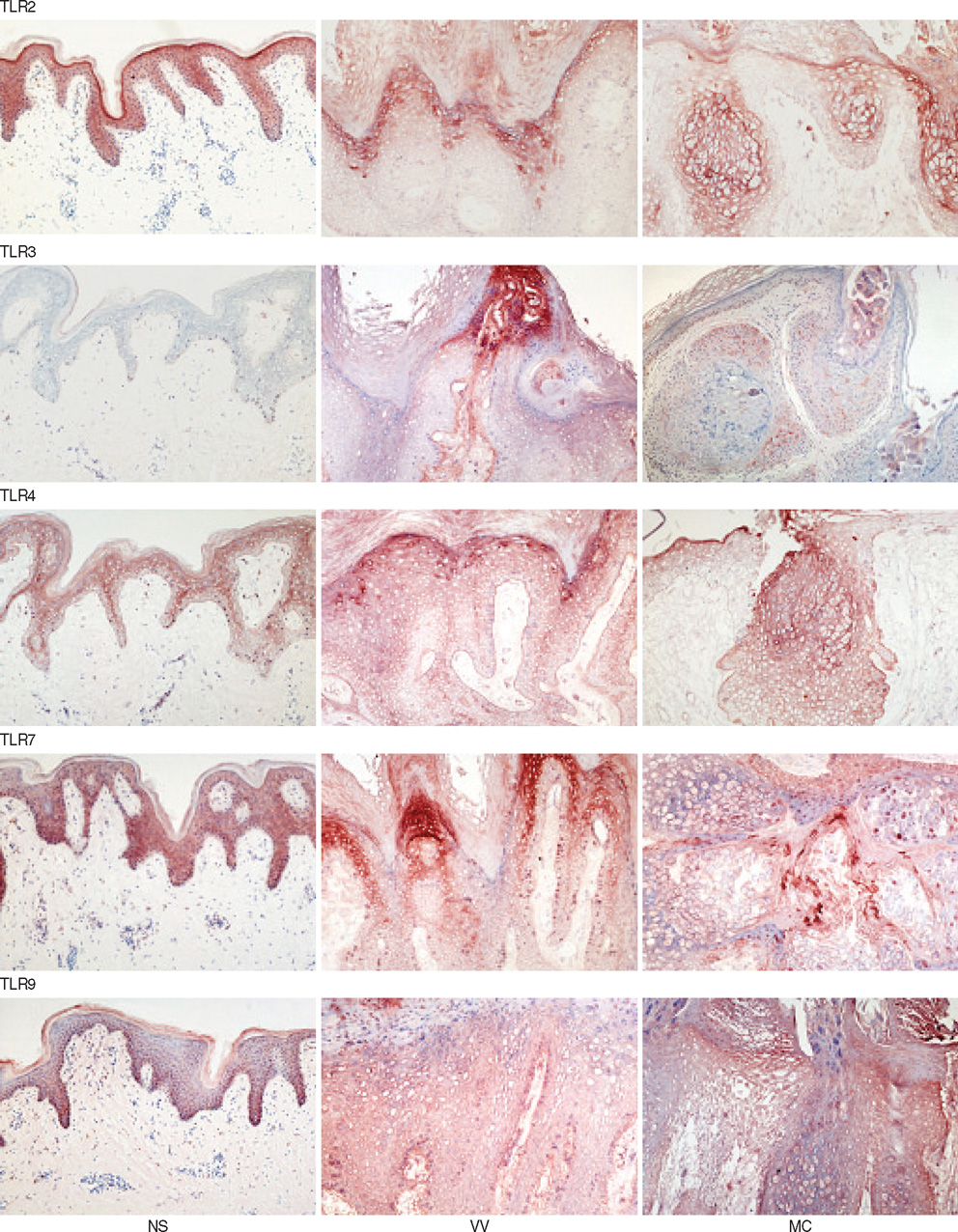
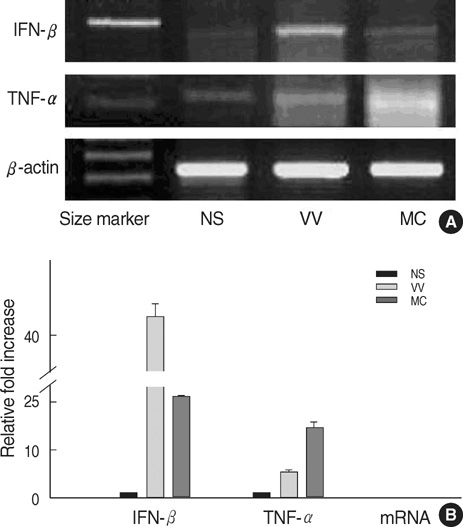
- Recent studies indicate that several Toll-like receptors (TLRs) are implicated in recognizing viral structures and instigating immune responses against viral infections. The aim of this study is to examine the expression of TLRs and proinflammatory cytokines in viral skin diseases such as verruca vulgaris (VV) and molluscum contagiosum (MC). Reverse transcription-polymerase chain reaction and immunostaining of skin samples were performed to determine the expression of specific antiviral and proinflammatory cytokines as well as 5 TLRs (TLR2, 3, 4, 7, and 9). In normal human skin, TLR2, 4, and 7 mRNA was constitutively expressed, whereas little TLR3 and 9 mRNA was detected. Compared to normal skin (NS), TLR3 and 9 mRNA was clearly expressed in VV and MC specimens. Likewise, immunohistochemistry indicated that keratinocytes in NS constitutively expressed TLR2, 4, and 7; however, TLR3 was rarely detected and TLR9 was only weakly expressed, whereas 5 TLRs were all strongly expressed on the epidermal keratinocytes of VV and MC lesions. In addition, the mRNA expression of IFN-beta and TNF-alpha was upregulated in the VV and MC samples. Immunohistochemistry indicated that IFN-beta and TNF-alpha were predominately localized in the granular layer in the VV lesions and adjacent to the MC bodies. Our results indicated that VV and MC skin lesions expressed TLR3 and 9 in addition to IFN-beta and TNF-alpha. These viral-induced proinflammatory cytokines may play a pivotal role in cutaneous innate immune responses.
Keyword
MeSH Terms
-
Cytokines/metabolism
*Gene Expression Regulation
Humans
Immunohistochemistry/methods
Inflammation
Interferon-beta/biosynthesis
Keratinocytes/cytology
Models, Biological
Molluscum Contagiosum/*metabolism
Toll-Like Receptor 3/biosynthesis
Toll-Like Receptor 9/biosynthesis
Toll-Like Receptors/*biosynthesis
Tumor Necrosis Factor-alpha/biosynthesis
Warts/*metabolism
Figure
Cited by 2 articles
-
Topical ALA-Photodynamic Therapy for Acne Can Induce Apoptosis of Sebocytes and Down-regulate Their TLR-2 and TLR-4 Expression
Eugene Jeong, Ji Won Hong, Jung Ah Min, Dong Won Lee, Mi Yeung Sohn, Weon Ju Lee, Jun Young Lee, Young Min Park
Ann Dermatol. 2011;23(1):23-32. doi: 10.5021/ad.2011.23.1.23.Attenuated Nuclear Factor Kappa B Activity by E7 Protein of Human Papillomavirus Type 2 in Human Epidermal Keratinocytes
Yun Hee Ryu, Ju Hee Han, Ji Hyun Lee, Soon Young Choi, Young Min Park
Ann Dermatol. 2017;29(3):367-370. doi: 10.5021/ad.2017.29.3.367.
Reference
-
1. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila toll. Proc Natl Acad Sci USA. 1998. 95:588–593.
Article2. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000. 406:782–787.
Article3. Ulevitch RJ, Mathison JC, da Silva Correia J. Innate immune responses during infection. Vaccine. 2004. 22:Suppl 1. S25–S30.
Article4. Ulevitch RJ. Therapeutics targeting the innate immune system. Nat Rev Immunol. 2004. 4:512–520.
Article5. Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999. 163:1–5.6. Morrison LA. The toll of herpes simplex virus infection. Trends Microbiol. 2004. 12:353–356.
Article7. Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002. 293:1364–1369.
Article8. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002. 3:196–200.
Article9. Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003. 198:513–520.
Article10. Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003. 102:956–963.
Article11. Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem Biophys Res Commun. 2000. 273:1161–1167.
Article12. Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-kappa B through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Bio Chem. 1999. 274:7611–7614.13. Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002. 30:185–194.
Article14. Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, Armstrong CA, Ansel JC. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002. 119:424–432.
Article15. Mempel M, Voelcker V, Kollisch G, Plank C, Rad R, Gerhard M, Schnopp C, Fraunberger P, Walli AK, Ring J, Abeck D, Ollert M. Toll-like receptor expression in human keratinocytes: Nuclear Factor κB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003. 121:1389–1396.
Article16. Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express toll-like receptors 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003. 148:670–679.17. Fiers W, Remaut E, Devos R, Cheroutre H, Contreras R, Gheysen D, Degrave W, Stanssens P, Tavernier J, Taya Y, Content J. The human fibroblast and human immune interferon genes and their expression in homologous and heterologous cells. Philos Trans R Soc Lond B Biol Sci. 1982. 299:29–38.18. Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins SF, Jordan H, Moore T, Max SI, Wang SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA; Mammalian Gene Collection Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002. 99:16899–16903.19. Triantafilou K, Vakakis E, Orthopoulos G, Ahmed MA, Schumann C, Lepper PM, Triantafilou M. TLR8 and TLR7 are involved in the host's immune response to human parechovirus 1. Eur J Immunol. 2005. 35:2416–2423.
Article20. Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, Beer Z, Beta-Csorgoo Z, Magocsi M, Rajnavolgyi E, Dobozy A, Kemeny L. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003. 15:721–730.
Article21. Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, Bauer S, Jakob T, Mempel M, Ollert M. Various members of Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005. 114:531–541.22. Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, de Jong EC. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007. 127:331–341.
Article23. Lowy DR, Androphy EJ. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Molluscum contagiosum. Fitzpatrick's Dermatology in general medicine. 2003. 6th ed. New York: McGraw-hill;2114–2117.24. Lowy DR, Androphy EJ. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Warts. Fitzpatrick's Dermatology in general medicine. 2003. 6th ed. New York: McGraw-hill;2119–2131.25. Grassegger A, Rollinger-Holzinger I, Zelger BW, Heim K, Zwierzina H, Fritsch PO, Hopfl RM. Spontaneous or interferon-gamma-induced T-cell infiltration, HLA-DR and ICAM-1 expression in genitoanal warts are associated with TH1 or mixed TH1/TH2 cytokine mRNA expression profiles. Arch Dermatol Res. 1997. 289:243–250.26. Jackson M, McKenzie RC, Benton EC, Hunter JA, Norval M. Cytokine mRNA expression in cutaneous warts: induction of interleukin-1 alpha. Arch Dermatol Res. 1996. 289:28–34.27. Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003. 171:4320–4328.
Article28. Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005. 79:3350–3357.
Article29. Hengge UR, Ruzicka T. Topical immunomodulation in dermatology: potential of toll-like receptor agonists. Dermatol Surg. 2004. 30:1101–1112.
Article30. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001. 413:732–738.31. Doyle SE, O'Connell R, Vaidya SA, Chow EK, Yee K, Cheng G. Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4. J Immunol. 2003. 170:3565–3571.
Article32. Tissari J, Siren J, Meri S, Julkunen I, Matikainen S. IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulaing TLR3 expression. J Immunol. 2005. 174:4289–4294.33. Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol Immunol. 2004. 48:147–154.
Article34. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004. 5:987–995.
Article35. Lebre MC, Antons TC, Kalinski P, Schuitemaker JH, van Capel TM, Kapsenberg ML, de Jong EC. Double-stranded RNA-exposed human keratinocytes promote Th1 responses by inducing a Type-1 polarized phenotype in dendritic cells: role of keratinocyte-derived tumor necrosis factor alpha, type I interferons, and interleukin-18. J Invest Dermatol. 2003. 120:990–997.36. Harte MT, Haga IR, Maloney G, Gray P, Reading PC, Bartlett NW, Smith GL, Bowie A, O'Neill LA. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med. 2003. 197:343–351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molluscum Contagiosum Virus Infection in an Epidermal Cyst
- Molluscum contagiosum occuring in an epidermal cyst
- A Case of Molluscum Contagiosum Treated by Ingenol Mebutate (Picato®)
- A Case of Lichen Nitidus Coexisted with Molluscum Contagiosum
- Serum IgE Level in Patients of Atopic Dermatitis and Atopic Dermatitis with Molluscum Contagiosum