J Korean Med Sci.
2007 Oct;22(5):868-872. 10.3346/jkms.2007.22.5.868.
Treatment of Facial Seborrheic Dermatitis with Pimecrolimus Cream 1%: An Open-Label Clinical Study in Korean Patients
- Affiliations
-
- 1Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea.
- 2Department of Dermatology, Pusan National University School of Medicine, Busan, Korea. drkmp@hanmail.net
- 3Mok Hye-Su, Jang Ho-Sun Dermatology Clinic, Busan, Korea.
- 4Medical Research Institute, Pusan National University, Busan, Korea.
- KMID: 1713295
- DOI: http://doi.org/10.3346/jkms.2007.22.5.868
Abstract
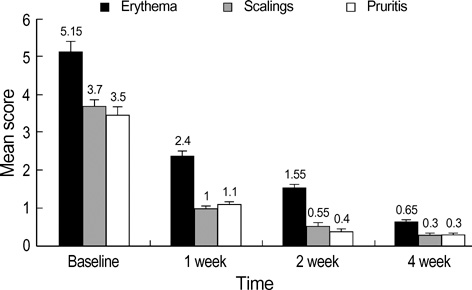
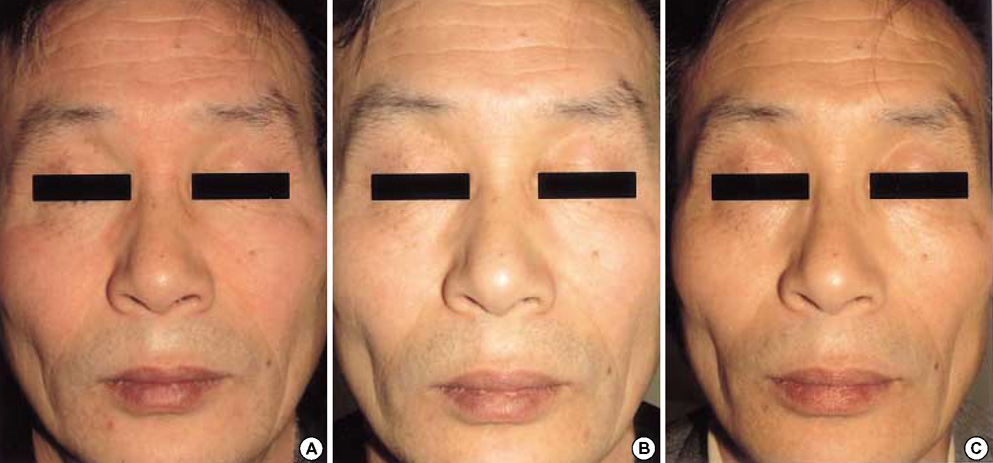
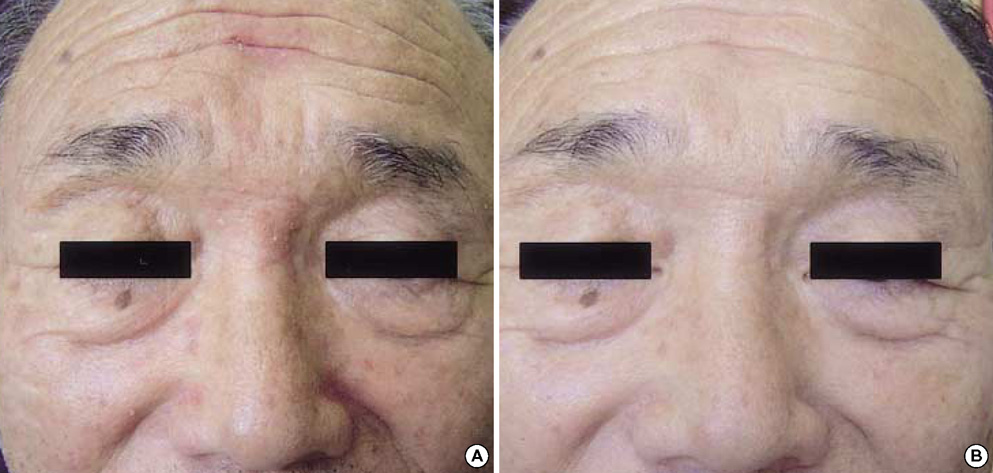
- Pimecrolimus cream 1% has shown to be effective in patients with a variety of inflammatory cutaneous disorders. And it might be a useful modality in the treatment of seborrheic dermatitis. This prospective study was aimed at assessing the efficacy and tolerability of pimecrolimus cream 1% in the treatment of facial seborrheic dermatitis. Twenty patients were instructed to apply pimecrolimus cream 1% for 4 consecutive weeks. Assessment of the disease severity was performed at baseline and at week 1, 2, and 4. Clinical assessments of erythema, scaling, and pruritus were measured using a 4-point scale (0-3). Global assessments of the disease severity by patients and investigators were performed at each visit. Mean clinical scores of erythema, scaling, and pruritus significantly improved by 87.4%, 91.9%, and 91.5% respectively at week 4 (p<0.001). Improvements in the global assessment of disease severity determined by patients and investigators also showed excellent results. No specific adverse events other than transient burning and tingling sensations were noted. The relapse of facial seborrheic dermatitis was mostly observed between 3 to 8 weeks after the discontinuation of pimecrolimus. We suggest that the topical application of pimecrolimus cream 1% can be an effective and safe alternative for treatment of facial seborrheic dermatitis.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Maintenance Therapy of Facial Seborrheic Dermatitis with 0.1% Tacrolimus Ointment
Hye One Kim, Yoon Seok Yang, Hyun Chang Ko, Gyung Moon Kim, Sang Hyun Cho, Young Joon Seo, Sang Wook Son, Jong Rok Lee, Joong Sun Lee, Sung Eun Chang, Jae We Che, Chun Wook Park
Ann Dermatol. 2015;27(5):523-530. doi: 10.5021/ad.2015.27.5.523.
Reference
-
1. Fritsch PO, Reider N. Bolognia JL, Jorizzo JL, Rapini RP, editors. Other Eczematous Eruption. Dermatology. 2003. Philadelphia: Mosby;215–226.2. Plewig G, Jansen T. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Seborrheic Dermatitis. Fitzpatrick's Dermatology in General medicine. 2003. New York: McGraw-Hill;1198–1204.3. Green CA, Farr PM, Shuster S. Treatment of seborrhoeic dermatitis with ketoconazole: II. Response of seborrhoeic dermatitis of the face, scalp and trunk to topical ketoconazole. Br J Dermatol. 1987. 116:217–221.
Article4. Pierard-Franchimont C, Pierard GE. A double-blind placebo-controlled study of ketoconazole+desonide gel combination in the treatment of facial seborrheic dermatitis. Dermatology. 2002. 204:344–347.5. Faergemann J. Management of seborrheic dermatitis and pityriasis versicolor. Am J Clin Dermatol. 2000. 1:75–80.
Article6. Park EJ, Kim CW, Kim KH, Ahn KJ, Kim KJ. Therapeutic effect of itraconazole in seborrheic dermatitis. Korean J Med Mycol. 2004. 9:222–229.7. Gupta AK, Chow M. Pimecrolimus: a review. J Eur Acad Dermatol Venereol. 2003. 17:493–503.
Article8. Crutchfield CE 3rd. Pimecrolimus: a new treatment for seborrheic dermatitis. Cutis. 2002. 70:207–208.9. Rigopoulos D, Ioannides D, Kalogeromitros D, Gregoriou S, Katsambas A. Pimecrolimus cream 1% vs. betamethasone 17-valerate 0.1% cream in the treatment of seborrhoeic dermatitis. A randomized open-label clinical trial. Br J Dermatol. 2004. 151:1071–1075.
Article10. Rallis E, Nasiopoulou A, Kouskoukis C, Koumantaki E. Pimecrolimus cream 1% can be an effective treatment for seborrheic dermatitis of the face and trunk. Drugs Exp Clin Res. 2004. 30:191–195.11. Brownell I, Quan LT, Hsu S. Topical pimecrolimus in the treatment of seborrheic dermatitis. Dermatol Online J. 2003. 9:13.12. Braza TJ, DiCarlo JB, Soon SL, McCall CO. Tacrolimus 0.1% ointment for seborrhoeic dermatitis: an open-label pilot study. Br J Dermatol. 2003. 148:1242–1244.
Article13. Meshkinpour A, Sun J, Weinstein G. An open pilot study using tacrolimus ointment in the treatment of seborrheic dermatitis. J Am Acad Dermatol. 2003. 49:145–147.
Article14. Faergemann J, Jones JC, Hettler O, Loria Y. Pityrosporum ovale (Malassezia furfur) as the causative agent of seborrheic dermatitis: new treatment options. Br J Dermatol. 1996. 134:Suppl 46. 12–15.15. Neuber K, Kroger S, Gruseck E, Abeck D, Ring J. Effects of Pityrosporum ovale on proliferation, immunoglobulin (IgA, G, M) synthesis and cytokine (IL-2, IL-10, IFN gamma) production of peripheral blood mononuclear cells from patients with seborrhoeic dermatitis. Arch Dermatol Res. 1996. 288:532–536.16. Faergemann J. Pityrosporum species as a cause of allergy and infection. Allergy. 1999. 54:413–419.17. Sugita T, Tajima M, Ito T, Saito M, Tsuboi R, Nishikawa A. Anti-fungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J Clin Microbiol. 2005. 43:2824–2829.
Article18. Eichenfield LF, Lucky AW, Boguniewicz M, Langley RG, Cherill R, Marshall K, Bush C, Graeber M. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002. 46:495–504.
Article19. Queille-Roussel C, Paul C, Duteil L, Lefebvre MC, Rapatz G, Zagula M, Ortonne JP. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for 4 weeks: a randomized, double-blind controlled study. Br J Dermatol. 2001. 144:507–513.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three Cases of Facial Atopic Dermatitis Treated with Topical Pimecrolimus (Elidel)
- Efficacy and Safety of Cream Containing Climbazole/Piroctone Olamine for Facial Seborrheic Dermatitis: A Single-Center, Open-Label Split-Face Clinical Study
- Two Cases of Interdigital Psoriasis Successfully Treated with Pimecrolimus Cream 1%
- A Pilot Study of 1% Pimecrolimus Cream for the Treatment of Childhood Segmental Vitiligo
- Pityrosporum(Malassezia) Related Diseases Especially Seborrheic Dermatitis