J Korean Med Sci.
2007 Oct;22(5):839-845. 10.3346/jkms.2007.22.5.839.
The Combination of Tiotropium and Budesonide in the Treatment of Chronic Obstructive Pulmonary Disease
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kangnam General Hospital, Yongin, Korea.
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. ysshim@snu.ac.kr
- 3Lung Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1713290
- DOI: http://doi.org/10.3346/jkms.2007.22.5.839
Abstract
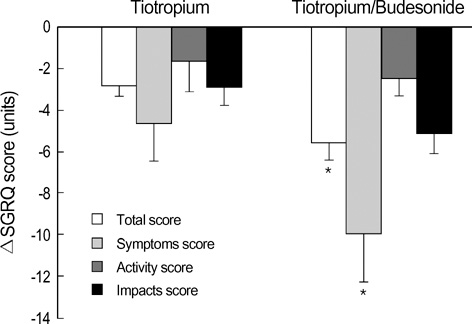
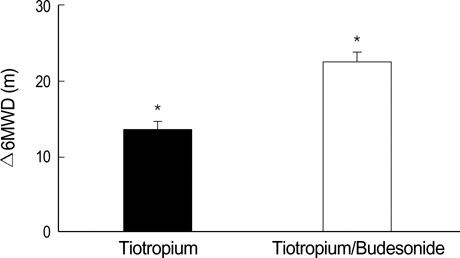
- Because additive effects of inhaled corticosteroids and long-acting anticholinergics are unclear, we undertook this study to compare the efficacy of tiotropium alone and tiotropium plus budesonide in patients with chronic obstructive pulmonary disease. The study subjects were randomized to receive either tiotropium 18 microgram once daily with or without budesonide 200 microgram twice daily for 6 weeks. The efficacy variables were changes in trough forced expiratory volume in one second (FEV1), St. George's Respiratory Questionnaire (SGRQ), 6-minute walk distance (6MWD), and use of rescue medication. One hundred patients were randomized and 81 completed the study. The mean age was 64.0 yr, and the mean FEV1 was 39.7% predicted. Compared with tiotropium alone (N=40), the tiotropium/budesonide combination (N=41) was related to an improvement in the SGRQ total score (tiotropium -2.8 units and tiotropium/budesonide -5.6 units, p=0.003). 6MWD was improved by 13.5 m in the tiotropium group and by 22.5 m in the tiotropium/budesonide group (p=0.031). Changes in trough FEV1 and the use of rescue medication were similar between two groups. In conclusion, compared with tiotropium alone, the tiotropium/ budesonide combination was related to an improved health-related quality of life. These data support that low-dose budesonide may enhance the efficacy of tiotropium.
Keyword
MeSH Terms
-
Aged
Bronchodilator Agents/*administration & dosage
Budesonide/*administration & dosage
Exercise
Female
Humans
Male
Middle Aged
Models, Statistical
Pulmonary Disease, Chronic Obstructive/*drug therapy
Quality of Life
Questionnaires
Scopolamine Derivatives/*administration & dosage
Spirometry/methods
Treatment Outcome
Figure
Reference
-
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Update of the management sections. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. 2001. accessed: 23 Sep 2006. Bethesda: National Heart, Lung and Blood Institute;http://www.goldcopd.com.2. Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD a summary of the ATS/ERS position paper. Eur Respir J. 2004. 23:932–946.
Article3. Disse B, Speck GA, Rominger KL, Witek TJ Jr, Hammer R. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999. 64:457–464.
Article4. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003. 58:399–404.
Article5. Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ Jr, Kesten S, Towse L. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002. 122:47–55.
Article6. Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, Cornelissen PJ. Dutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002. 19:209–216.7. Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek T Jr. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002. 19:217–224.
Article8. Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA Jr, Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005. 143:317–326.9. van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000. 55:289–294.10. Oostenbrink JB, Rutten-van Molken MP, Al MJ, Van Noord JA, Vincken W. One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. Eur Respir J. 2004. 23:241–249.11. Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005. 127:809–817.
Article12. Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, di Marco F, Matera MG. The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respir Med. 2004. 98:1214–1221.
Article13. van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Mueller A, Cornelissen PJ. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005. 26:214–222.
Article14. van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006. 129:509–517.
Article15. Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000. 161:342–344.
Article16. Highland KB. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a long-term benefit? Curr Opin Pulm Med. 2004. 10:113–119.17. Miravitlles M, Mayordomo C, Artes M, Sanchez-Agudo L, Nicolau F, Segu JL. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice. EOLO Group. Estudio Observacional de la Limitacion Obstructiva al Flujo aEreo. Respir Med. 1999. 93:173–179.18. Van Andel AE, Reisner C, Menjoge SS, Witek TJ. Analysis of inhaled corticosteroid and oral theophylline use among patients with stable COPD from 1987 to 1995. Chest. 1999. 115:703–707.
Article19. van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002. 166:1358–1363.20. Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002. 166:1084–1091.
Article21. Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003. 361:449–456.
Article22. Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003. 21:74–81.
Article23. Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, Shah T. The efficacy and safety of fluticasone propionate (250 microg)/ salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003. 124:834–843.24. Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003. 22:912–919.25. Emala CW, Clancy J, Hirshman CA. Glucocorticoid treatment decreases muscarinic receptor expression in canine airway smooth muscle. Am J Physiol. 1997. 272:L745–L751.
Article26. Jacoby DB, Yost BL, Kumaravel B, Chan-Li Y, Xiao HQ, Kawashima K, Fryer AD. Glucocorticoid treatment increases inhibitory m(2) muscarinic receptor expression and function in the airways. Am J Respir Cell Mol Biol. 2001. 24:485–491.27. American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995. 152:1107–1136.28. Jones PW, Quirk FH, Baveystock CM. The St Georges Respiratory Questionnaire. Respir Med. 1991. 85:Suppl B. 25–31.
Article29. Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001. 56:880–887.
Article30. Kim EJ, Park JH, Yoon SJ, Lee SJ, Cha SI, Park JY, Jung TH, Kim CH. Relationship between dyspnea and disease severity, quality of life, and social factors in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2006. 60:397–403.31. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002. 166:111–117.32. Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997. 155:1278–1282.
Article33. Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2003. 58:937–941.
Article34. Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006. 27:547–555.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Tiotropium+Olodaterol Dual Bronchodilator Therapy in the Management of Chronic Obstructive Pulmonary Disease
- Issues on Safety of Long-Acting Muscarinic Antagonist
- Effect of inhaled tiotropium on patients with COPD
- The Effects of Different Delivery Methods of Inhaled Budesonlde on the Plasma Cortisol Concentration
- Optimal Bronchodilation for COPD Patients: Are All Long-Acting β₂-Agonist/Long-Acting Muscarinic Antagonists the Same?