J Korean Med Sci.
2006 Dec;21(6):973-978. 10.3346/jkms.2006.21.6.973.
Three-Year Follow-up of an Outbreak of Serratia marcescens Bacteriuria in a Neurosurgical Intensive Care Unit
- Affiliations
-
- 1Department of Internal Medicine, Inje University, Sanggyepaik Hospital, 761-1 Sanggye 7-dong, Nowon-gu, Seoul, Korea. kimbn@sanggyepaik.ac.kr
- 2Infection Control Unit, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 3Department Laboratory Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- KMID: 1713103
- DOI: http://doi.org/10.3346/jkms.2006.21.6.973
Abstract
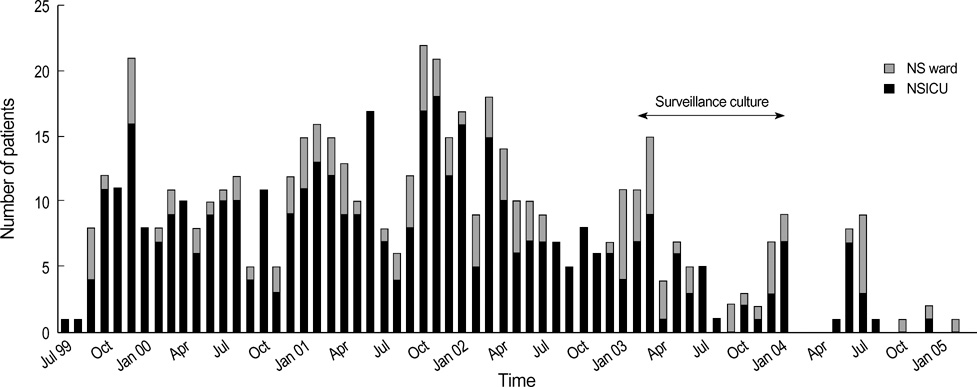
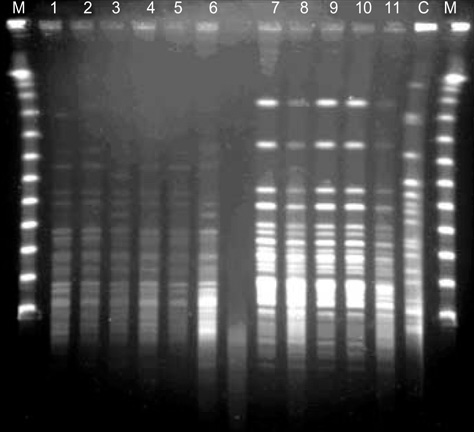
- We report on the investigations and interventions conducted to contain an extended outbreak of Serratia marcescens bacteriuria that lasted for years in a neurosurgical intensive care unit (NSICU). A case-control study was performed to identify the risk factors for S. marcescens acquisition in urine. In case patients, urine sampling for tests and central venous catheterization were performed more frequently before the isolation of S. marcescens. Case patients were more frequently prescribed third-generation cephalosporins. Adherence to hand antisepsis was encouraged through in-service educational meetings and infection control measures, especially concerning the manipulation of indwelling urinary catheters, were intensified. The outbreak persisted despite the reinforcement of infection control measures. However, no patient has newly acquired the organism in the NSICU since December 2004. Multiple factors, including inadequate infection control practices and inappropriate antimicrobial usage, possibly contributed to the persistence of this S. marcescens outbreak. Healthcare workers should consistently follow infection control policies to ensure quality care.
MeSH Terms
-
Serratia Infections/*epidemiology/*prevention & control/transmission
Risk Factors
Risk Assessment/*methods
Population Surveillance
Neurosurgery/*statistics & numerical data
Middle Aged
Male
Korea/epidemiology
Intensive Care Units/*statistics & numerical data
Infection Control/methods/statistics & numerical data
Incidence
Humans
Follow-Up Studies
Female
Disease Transmission, Horizontal/prevention & control/statistics & numerical data
Disease Outbreaks/prevention & control/statistics & numerical data
Case-Control Studies
Bacteriuria/*epidemiology/*prevention & control
Figure
Reference
-
1. Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979. 300:887–893.2. Acar JF. Serratia marcescens infections. Infect Control. 1986. 7:273–278.
Article3. Wilfert JN, Barrett FF, Kass EH. Bacteremia due to Serratia marcescens. N Engl J Med. 1968. 279:286–289.4. Sanders CC, Watanakunakorn C. Emergence of resistance to beta-lactams, aminoglycosides, and quinolones during combination therapy for infection due to Serratia marcescens. J Infect Dis. 1986. 153:617–619.5. Herra CM, Knowles SJ, Kaufmann ME, Mulvihill E, McGrath B, Keane CT. An outbreak of an unusual strain of Serratia marcescens in two Dublin hospitals. J Hosp Infect. 1998. 39:135–141.
Article6. Pagani L, Luzzaro F, Ronza P, Rossi A, Micheletti P, Porta F, Romero E. Outbreak of extended-spectrum beta-lactamase producing Serratia marcescens in an intensive care unit. FEMS Immunol Med Microbiol. 1994. 10:39–46.7. Schaberg DR, Alford RH, Anderson R, Farmer JJ III, Melly MA, Schaffner W. An outbreak of nosocomial infection due to multiply resistant Serratia marcescens: evidence of interhospital spread. J Infect Dis. 1976. 134:181–188.
Article8. Bagattini M, Crispino M, Gentile F, Barretta E, Schiavone D, Boccia MC, Triassi M, Zarrilli R. A nosocomial outbreak of Serratia marcescens producing inducible Amp C-type beta-lactamase enzyme and carrying antimicrobial resistance genes within a class 1 integron. J Hosp Infect. 2004. 56:29–36.
Article9. Su LH, Ou JT, Leu HS, Chiang PC, Chiu YP, Chia JH, Kuo AJ, Chiu CH, Chu C, Wu TL, Sun CF, Riley TV, Chang BJ. Extended epidemic of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol. 2003. 41:4726–4732.
Article10. Farmer JJ III, Davis BR, Hickman FW, Presley DB, Bodey GP, Negut M, Bobo RA. Detection of Serratia outbreaks in hospital. Lancet. 1976. 2:455–459.
Article11. Newport MT, John JF, Michel YM, Levkoff AH. Endemic Serratia marcescens infection in a neonatal intensive care nursery associated with gastrointestinal colonization. Pediatr Infect Dis. 1985. 4:160–167.
Article12. Christensen GD, Korones SB, Reed L, Bulley R, McLaughlin B, Bisno AL. Epidemic Serratia marcescens in a neonatal intensive care unit: importance of the gastrointestinal tract as a reservoir. Infect Control. 1982. 3:127–133.
Article13. Maki DG, Hennekens CG, Phillips CW, Shaw WV, Bennett JV. Nosocomial urinary tract infection with Serratia marcescens: an epidemiologic study. J Infect Dis. 1973. 128:579–587.
Article14. Madduri SD, Mauriello DA, Smith LG, Seebode JJ. Serratia marcescens and the urologist. J Urol. 1976. 116:613–615.
Article15. Cann KJ, Johnstone D, Skene AI. An outbreak of Serratia marcescens infection following urodynamic studies. J Hosp Infect. 1987. 9:291–293.
Article16. Krieger JN, Levy-Zombek E, Scheidt A, Drusin LM. A nosocomial epidemic of antibiotic-resistant Serratia marcescens urinary tract infections. J Urol. 1980. 124:498–502.
Article17. Okuda T, Endo N, Osada Y, Zen-Yoji H. Outbreak of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol. 1984. 20:691–695.
Article18. Rutala WA, Kennedy VA, Loflin HB, Sarubbi FA Jr. Serratia marcescens nosocomial infections of the urinary tract associated with urine measuring containers and urinometers. Am J Med. 1981. 70:659–663.
Article19. Echols RM, Palmer DL, King RM, Long GW. Multidrug-resistant Serratia marcescens bacteriuria related to urologic instrumentation. South Med J. 1984. 77:173–177.
Article20. Arroyo JC, Milligan WL, Postic B, Northey J, Parker E, Bryan CS. Clinical, epidemiologic and microbiologic features of a persistent outbreak of amikacin-resistant Serratia marcescens. Infect Control. 1981. 2:367–372.
Article21. John JF Jr, McNeill WF. Characteristics of Serratia marcescens containing a plasmid coding for gentamicin resistance in nosocomial infections. J Infect Dis. 1981. 143:810–817.
Article22. Simor AE, Ramage L, Wilcox L, Bull SB, Bialkowska-Hobrzanska H. Molecular and epidemiologic study of multiresistant Serratia marcescens infections in a spinal cord injury rehabilitation unit. Infect Control. 1988. 9:20–27.
Article23. Koh EH, Kim S, Bae IG. Epidemiological investigation of an outbreak of Serratia marcescens urinary track infection in an intensive care unit using pulsed-field gel electrophoresis. Korean J Clin Microbiol. 2005. 8:34–40.24. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995. 33:2233–2239.
Article25. Rutala WA. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. Association for Professionals in Infection Control and Epidemiology, Inc. Am J Infect Control. 1996. 24:313–342.26. Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983. 11:28–36.
Article27. Kelly DF. Neurosurgical postoperative care. Neurosurg Clin N Am. 1994. 5:789–810.
Article28. Guggenbichler JP, Kofler J. Influence of third-generation cephalosporins on aerobic intestinal flora. J Antimicrob Chemother. 1984. 14:Suppl B. 67–70.
Article29. Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995. 8:557–584.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiologic Investigation of an Outbreak of Serratia marcescens Urinary Tract Infection in an Intensive Care Unit Using Pulsed-Field Gel Electrophoresis
- Clinical aspects of an outbreak of Serratia marcescens infections in neonates
- Skin Infection Caused by Serratia marcescens in an Immunocompetent Patient with Hidradenitis Suppurativa
- Clinical Bacteriologic Study of Serratia Marcescens Septicemia
- Serratia Marcescens Keratitis