J Vet Sci.
2013 Dec;14(4):487-490. 10.4142/jvs.2013.14.4.487.
Prolonged excretion of a low-pathogenicity H5N2 avian influenza virus strain in the Pekin duck
- Affiliations
-
- 1Departamentos de Medicina y Zootecnia de Aves, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autonoma de Mexico, Ciudad de Mexico 04510, Mexico. gary@unam.mx
- 2Departamentos de Biologia Molecular y Biotecnologia, Instituto de Investigaciones Biomedicas, Universidad Nacional Autonoma de Mexico, Ciudad de Mexico 04510, Mexico.
- 3Departamento de Biotecnologia en Salud Animal, Centro Nacional de Investigacion Disciplinaria en Microbiologia Animal, Instituto Nacional de Investigaciones Forestales, Agricolas y Pecuarias, Ciudad de Mexico 05110, Mexico.
- KMID: 1712317
- DOI: http://doi.org/10.4142/jvs.2013.14.4.487
Abstract
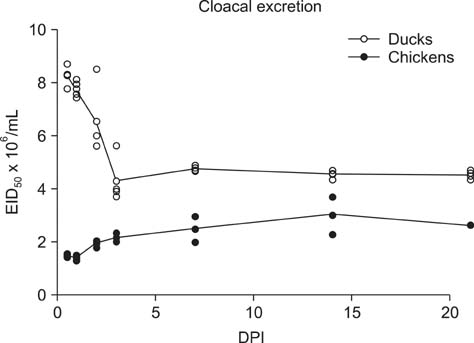
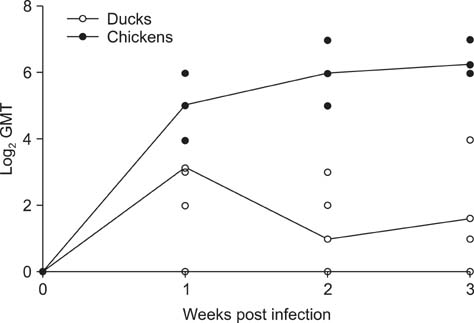
- H5N2 strains of low-pathogenicity avian influenza virus (LPAIV) have been circulating for at least 17 years in some Mexican chicken farms. We measured the rate and duration of viral excretion from Pekin ducks that were experimentally inoculated with an H5N2 LPAIV that causes death in embryonated chicken eggs (A/chicken/Mexico/2007). Leghorn chickens were used as susceptible host controls. The degree of viral excretion was evaluated with real-time reverse transcriptase-polymerase chain reaction (RRT-PCR) using samples from oropharyngeal and cloacal swabs. We observed prolonged excretion from both species of birds lasting for at least 21 days. Prolonged excretion of LPAIV A/chicken/Mexico/2007 is atypical.
Keyword
MeSH Terms
-
Animals
Chickens
Cloaca/virology
*Ducks
Influenza A Virus, H5N2 Subtype/*physiology
Influenza in Birds/*physiopathology/virology
Oropharynx/virology
Poultry Diseases/physiopathology/virology
Real-Time Polymerase Chain Reaction/veterinary
Reverse Transcriptase Polymerase Chain Reaction/veterinary
Time Factors
*Virus Shedding
Figure
Reference
-
1. Dufour-Zavala L, Swayne DE, Glisson JR, Pearson JE, Reed WM, Jackwood MW, Woolcock PR, editors. A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens. 5th ed. Athens: American Association of Avian Pathologists;2008. p. 1–249.2. Hirst GK, Pons MW. Mechanism of influenza recombination. II. Virus aggregation and its effect on plaque formation by so-called noninfective virus. Virology. 1973; 56:620–631.3. Horimoto T, Rivera E, Pearson J, Senne D, Krauss S, Kawaoka Y, Webster RG. Origin and molecular changes associated with emergence of a highly pathogenic H5N2 influenza virus in Mexico. Virology. 1995; 213:223–230.
Article4. Jourdain E, Gunnarsson G, Wahlgren J, Latorre-Margalef N, Bröjer C, Sahlin S, Svensson L, Waldenström J, Lundkvist Å, Olsen B. Influenza virus in a natural host, the mallard: experimental infection data. PLoS One. 2010; 5:e8935.
Article5. Ladman BS, Rosenberger SC, Rosenberger JK, Pope CR, Gelb J Jr. Virulence of low pathogenicity H7N2 avian influenza viruses from the Delmarva peninsula for broiler and leghorn chickens and turkeys. Avian Dis. 2008; 52:623–631.
Article6. Lee CW, Suarez DL. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J Virol Methods. 2004; 119:151–158.
Article7. Mundt E, Gay L, Jones L, Saavedra G, Tompkins SM, Tripp RA. Replication and pathogenesis associated with H5N1, H5N2, and H5N3 low-pathogenic avian influenza virus infection in chickens and ducks. Arch Virol. 2009; 154:1241–1248.
Article8. Pillai SPS, Suarez DL, Pantin-Jackwood M, Lee CW. Pathogenicity and transmission studies of H5N2 parrot avian influenza virus of Mexican lineage in different poultry species. Vet Microbiol. 2008; 129:48–57.
Article9. Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002; 40:3256–3260.
Article10. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179.
Article11. Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murti KG. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978; 84:268–278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4
- Pathogenicity of clade 2.3.4.4 H5N6 highly pathogenic avian influenza virus in three chicken breeds from South Korea in 2016/2017
- Current situation and control strategies of H9N2 avian influenza in South Korea
- Pathogenicity of H5N8 virus in chickens from Korea in 2014
- The significance of avian influenza virus mouse-adaptation and its application in characterizing the efficacy of new vaccines and therapeutic agents