J Vet Sci.
2013 Dec;14(4):381-386. 10.4142/jvs.2013.14.4.381.
Expression of E-cadherin in pig kidney
- Affiliations
-
- 1Department of Anatomy, Ewha Womans University School of Medicine, Seoul 158-710, Korea. khhan@ewha.ac.kr
- 2Division of Biomedical Engineering, Columbia University, New York, NY 10027, USA.
- 3Department of Surgery, Ewha Womans University School of Medicine, Seoul 158-710, Korea.
- KMID: 1712303
- DOI: http://doi.org/10.4142/jvs.2013.14.4.381
Abstract
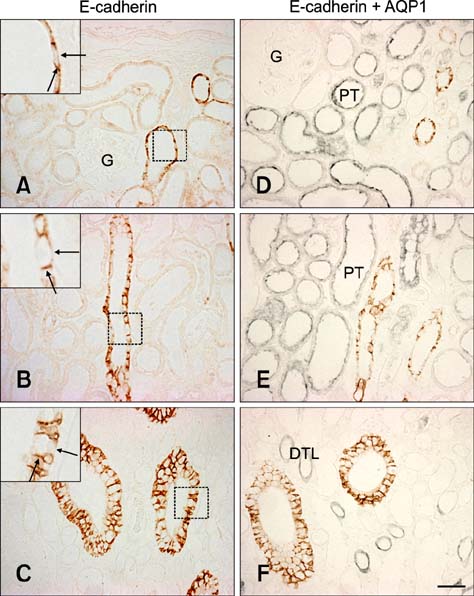
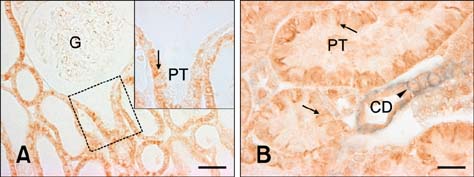
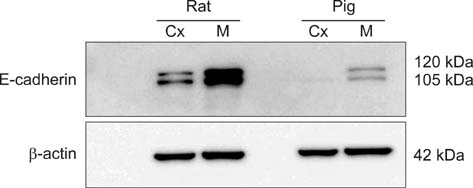
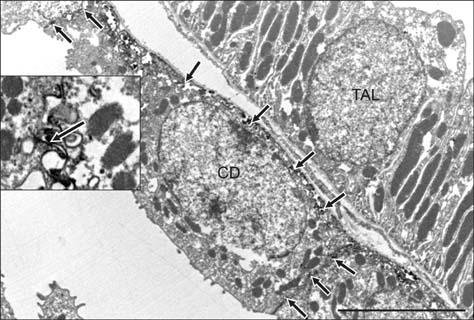
- E-cadherin is a cell adhesion molecule that plays an important role in maintaining renal epithelial polarity and integrity. The purpose of this study was to determine the exact cellular localization of E-cadherin in pig kidney. Kidney tissues from pigs were processed for light and electron microscopy immunocytochemistry, and immunoblot analysis. E-cadhedrin bands of the same size were detected by immunoblot of samples from rat and pig kidneys. In pig kidney, strong E-cadherin expression was observed in the basolateral plasma membrane of the tubular epithelial cells. E-cadherin immunolabeling was not detected in glomeruli or blood vessels of pig kidney. Double-labeling results demonstrated that E-cadherin was expressed in the calbindin D28k-positive distal convoluted tubule and H(+)-ATPase-positive collecting duct, but not in the aquaporin 1-positive, N-cadherin-positive proximal tubule. In contrast to rat, E-cadherin immunoreactivity was not expressed at detectable levels in the Tamm-Horsfall protein-positive thick ascending limb of pig kidney. Immunoelectron microscopy confirmed that E-cadherin was localized in both the lateral membranes and basal infoldings of the collecting duct. These results suggest that E-cadherin may be a critical adhesion molecule in the distal convoluted tubule and collecting duct cells of pig kidney.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Hydration status affects osteopontin expression in the rat kidney
Su-Youn Lee, Sae-Jin Lee, Hong-Lin Piao, Suk-Young Yang, I. David Weiner, Jin Kim, Ki-Hwan Han
J Vet Sci. 2016;17(3):269-277. doi: 10.4142/jvs.2016.17.3.269.
Reference
-
1. Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A. 1989; 86:5429–5433.
Article2. Bachmann S, Metzger R, Bunnemann B. Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle's loop in rat kidney. Histochemistry. 1990; 94:517–523.
Article3. Boller K, Vestweber D, Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985; 100:327–332.
Article4. Bush KT, Tsukamoto T, Nigam SK. Selective degradation of E-cadherin and dissolution of E-cadherin-catenin complexes in epithelial ischemia. Am J Physiol Renal Physiol. 2000; 278:F847–F852.5. Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963; 17:375–412.
Article6. Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994; 330:1580–1588.
Article7. Han KH, Woo SK, Kim WY, Park SH, Cha JH, Kim J, Kwon HM. Maturation of TonEBP expression in developing rat kidney. Am J Physiol Renal Physiol. 2004; 287:F878–F885.
Article8. Han KH, Lim JM, Kim WY, Kim H, Madsen KM, Kim J. Expression of endothelial nitric oxide synthase in developing rat kidney. Am J Physiol Renal Physiol. 2005; 288:F694–F702.
Article9. Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol. 2006; 17:2670–2679.
Article10. Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia-reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol. 2007; 293:F1342–F1354.
Article11. Jiang J, Dean D, Burghardt RC, Parrish AR. Disruption of cadherin/catenin expression, localization, and interactions during HgCl2-induced nephrotoxicity. Toxicol Sci. 2004; 80:170–182.
Article12. Kirk A, Campbell S, Bass P, Mason J, Collins J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant. 2010; 25:2107–2119.
Article13. Lee SY, Shin JA, Kwon HM, Weiner ID, Han KH. Renal ischemia-reperfusion injury causes intercalated cell-specific disruption of occludin in the collecting duct. Histochem Cell Biol. 2011; 136:637–647.
Article14. Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol. 1986; 250:F1–F15.
Article15. Molitoris BA, Falk SA, Dahl RH. Ischemia-induced loss of epithelial polarity: role of the tight junction. J Clin Invest. 1989; 84:1334–1339.
Article16. Nankivell BJ, Fenton-Lee CA, Kuypers DRJ, Cheung E, Allen RDM, O'Connell PJ, Chapman JR. Effect of histological damage on long-term kidney transplant outcome. Transplantation. 2001; 71:515–523.
Article17. Nielsen S, Agre P. The aquaporin family of water channels in kidney. Kidney Int. 1995; 48:1057–1068.
Article18. Nouwen EJ, Dauwe S, van der Biest I, de Broe ME. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int. 1993; 44:147–158.
Article19. Perantoni AO. Cell adhesion molecules in the kidney: from embryo to adult. Exp Nephrol. 1999; 7:80–102.
Article20. Piepenhagen PA, Peters LL, Lux SE, Nelson WJ. Differential expression of Na+-K+-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron. Am J Physiol. 1995; 269:C1417–C1432.21. Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004; 4:10.22. Prozialeck WC, Edwards JR. Cell adhesion molecules in chemically-induced renal injury. Pharmacol Ther. 2007; 114:74–93.
Article23. Roth J, Brown D, Norman AW, Orci L. Localization of the vitamin D-dependent calcium-binding protein in mammalian kidney. Am J Physiol. 1982; 243:F243–F252.
Article24. Tani T, Laitinen L, Kangas L, Lehto VP, Virtanen I. Expression of E- and N-cadherin in renal cell carcinomas, in renal cell carcinoma cell lines in vitro and in their xenografts. Int J Cancer. 1995; 64:407–414.
Article25. Thomson RB, Aronson PS. Immunolocalization of Ksp-cadherin in the adult and developing rabbit kidney. Am J Physiol. 1999; 277:F146–F156.26. Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998; 352:1691–1696.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of E-cadherin and a-catenin in Thyroid Carcinomas
- Prognostic Significance of E-cadherin Expression in Renal Cell Carcinoma
- E - cadherin Expression in Carcinoma of The Uterine Cervix
- Regulation of V-cadherin Expression on Human Dermal Microvascular Endothelial Cells
- Reduced Expression of E-cadherin in Human Non-small Cell Lung Carcinoma