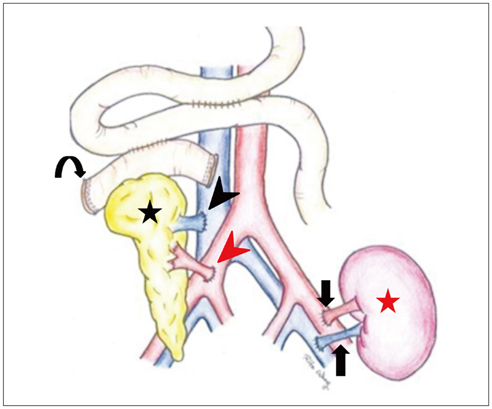
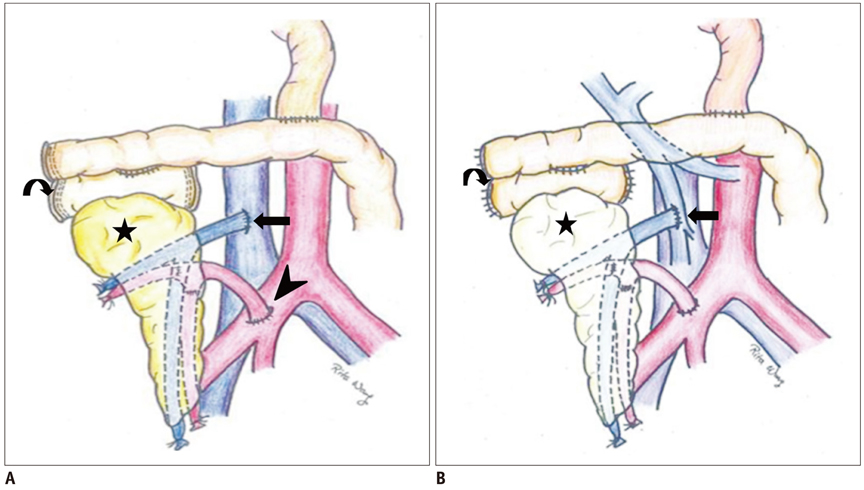
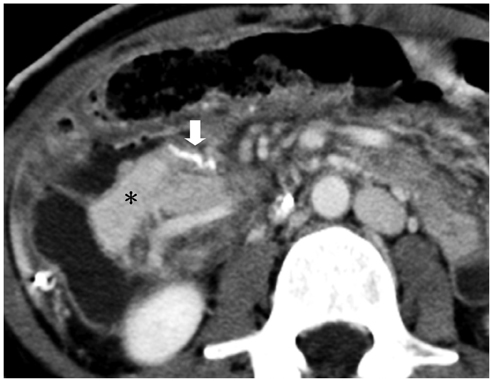
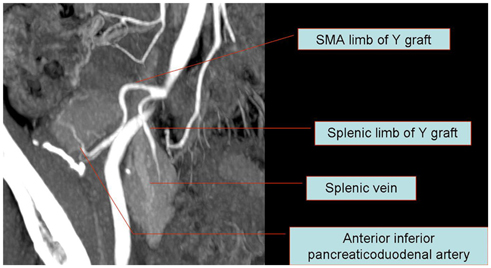
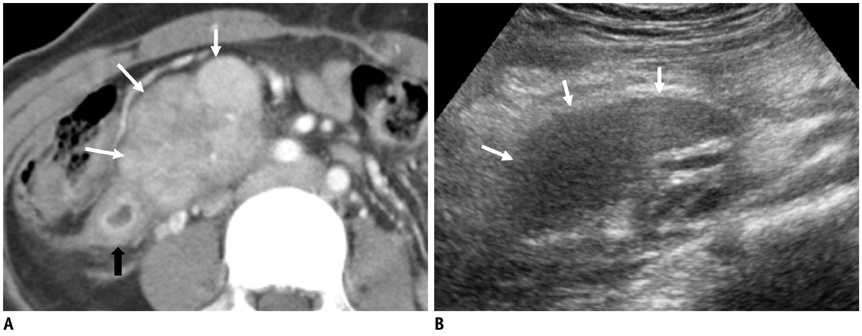
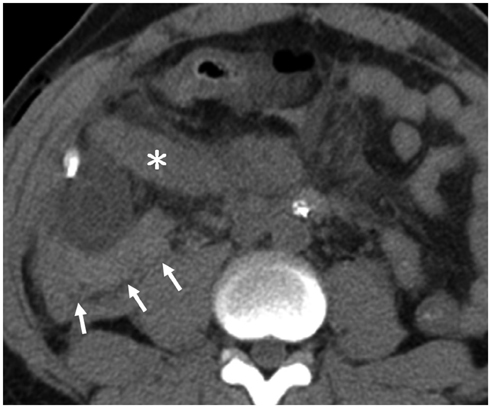
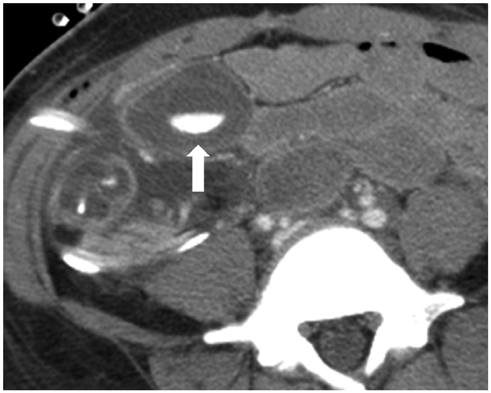
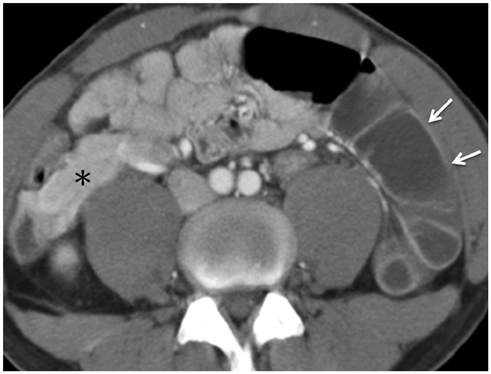
Korean J Radiol.
2014 Feb;15(1):45-53. 10.3348/kjr.2014.15.1.45.
Imaging Spectrum after Pancreas Transplantation with Enteric Drainage
- Affiliations
-
- 1Department of Radiology, Taichung Veterans General Hospital, Taichung 40705, Taiwan.
- 2Department of Radiology, Taipei Veterans General Hospital, Taipei 11217, Taiwan. rclee@vghtpe.gov.tw
- 3National Yang-Ming University School of Medicine, Taipei 11221, Taiwan.
- 4Department of Surgery, Taipei Veterans General Hospital, Taipei 11217, Taiwan.
- KMID: 1711476
- DOI: http://doi.org/10.3348/kjr.2014.15.1.45
Abstract
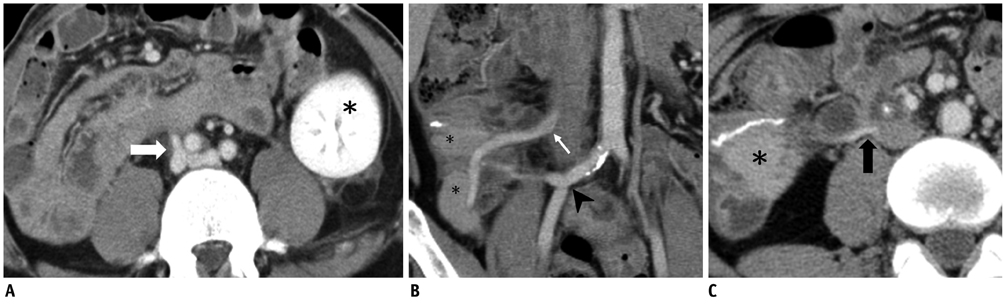
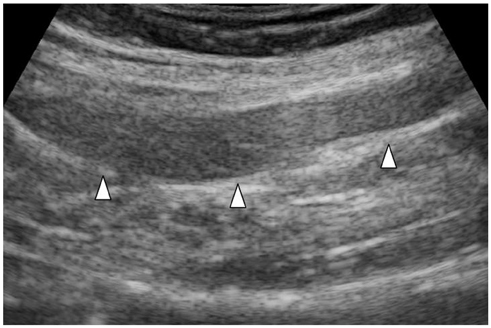
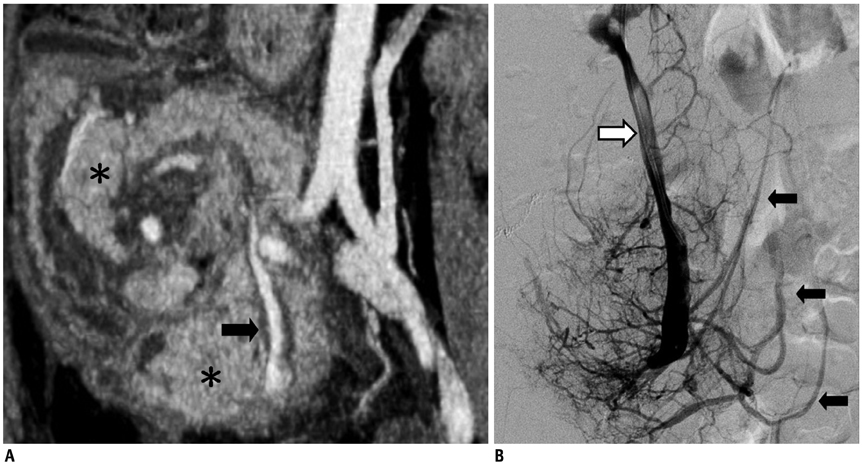
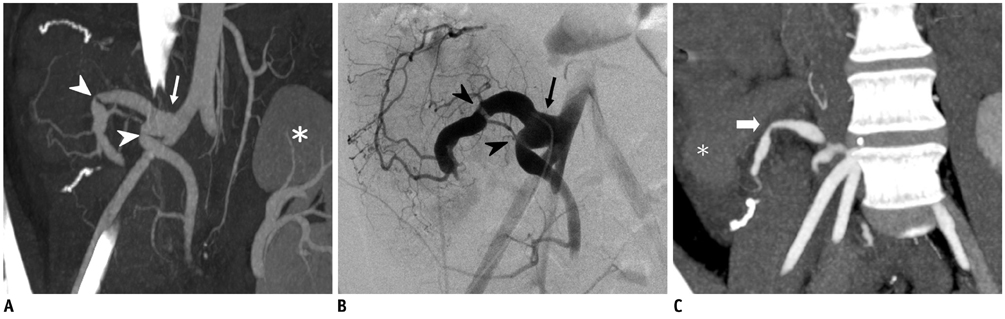
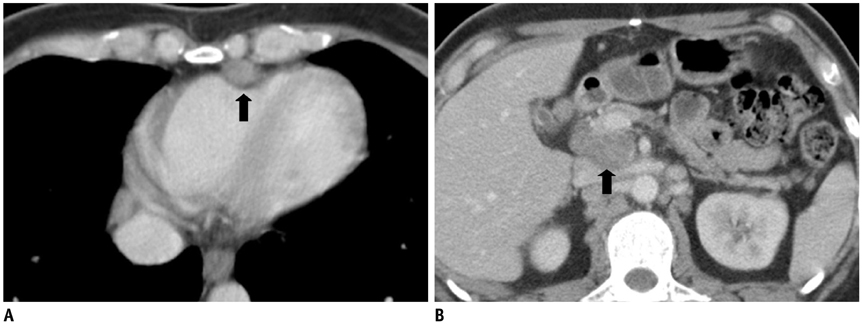
- Since the introduction of pancreas transplantation more than 40 years ago, surgical techniques and immunosuppressive regiments have improved and both have contributed to increase the number and success rate of this procedure. However, graft survival corresponds to early diagnosis of organ-related complications. Thus, knowledge of the transplantation procedure and postoperative image anatomy are basic requirements for radiologists. In this article, we demonstrate the imaging spectrum of pancreas transplantation with enteric exocrine drainage.
Keyword
MeSH Terms
-
Adult
Anastomosis, Surgical/methods
Diagnostic Imaging/methods
Drainage/methods
Female
Graft Rejection/pathology
Graft Survival
Humans
Iliac Artery/radiography/surgery
Immunosuppressive Agents
Kidney Transplantation
Male
*Medical Illustration
Mesenteric Artery, Superior/radiography/surgery
Middle Aged
Pancreas/*blood supply/radiography
Pancreas Transplantation/adverse effects/*methods
Pancreatitis, Graft/etiology
Portal Vein/radiography/surgery
Postoperative Complications/radiography
Postoperative Hemorrhage/etiology
Survival Rate
Immunosuppressive Agents
Figure
Reference
-
1. Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967; 61:827–837.2. Baktavatsalam R, Little DM, Connolly EM, Farrell JG, Hickey DP. Complications relating to the urinary tract associated with bladder-drained pancreatic transplantation. Br J Urol. 1998; 81:219–223.3. Vandermeer FQ, Manning MA, Frazier AA, Wong-You-Cheong JJ. Imaging of whole-organ pancreas transplants. Radiographics. 2012; 32:411–435.4. Hagspiel KD, Nandalur K, Pruett TL, Leung DA, Angle JF, Spinosa DJ, et al. Evaluation of vascular complications of pancreas transplantation with high-spatial-resolution contrast-enhanced MR angiography. Radiology. 2007; 242:590–599.5. Freund MC, Steurer W, Gassner EM, Unsinn KM, Rieger M, Koenigsrainer A, et al. Spectrum of imaging findings after pancreas transplantation with enteric exocrine drainage: Part 2, posttransplantation complications. AJR Am J Roentgenol. 2004; 182:919–925.6. Chandra J, Phillips RR, Boardman P, Gleeson FV, Anderson EM. Pancreas transplants. Clin Radiol. 2009; 64:714–723.7. Margreiter C, Mark W, Wiedemann D, Sucher R, Ollinger R, Bösmüller C, et al. Pancreatic graft survival despite partial vascular graft thrombosis due to splenocephalic anastomoses. Am J Transplant. 2010; 10:846–851.8. Drachenberg CB, Papadimitriou JC. Spectrum of histopathological changes in pancreas allograft biopsies and relationship to graft loss. Transplant Proc. 2007; 39:2326–2328.9. Holalkere NS, Soto J. Imaging of miscellaneous pancreatic pathology (trauma, transplant, infections, and deposition). Radiol Clin North Am. 2012; 50:515–528.10. Dillman JR, Elsayes KM, Bude RO, Platt JF, Francis IR. Imaging of pancreas transplants: postoperative findings with clinical correlation. J Comput Assist Tomogr. 2009; 33:609–617.11. Atwell TD, Gorman B, Larson TS, Charboneau JW, Ingalls Hanson BM, Stegall MD. Pancreas transplants: experience with 232 percutaneous US-guided biopsy procedures in 88 patients. Radiology. 2004; 231:845–849.12. Nelson NL, Largen PS, Stratta RJ, Taylor RJ, Grune MT, Hapke MR, et al. Pancreas allograft rejection: correlation of transduodenal core biopsy with Doppler resistive index. Radiology. 1996; 200:91–94.13. Aideyan OA, Foshager MC, Benedetti E, Troppmann C, Gruessner RW. Correlation of the arterial resistive index in pancreas transplants of patients with transplant rejection. AJR Am J Roentgenol. 1997; 168:1445–1447.14. Shyr YM, Su CH, Li AF, Wu CW, Lui WY. Canine pancreas allotransplantation with enteric drainage. Zhonghua Yi Xue Za Zhi (Taipei). 2002; 65:483–488.15. Farge D. Kaposi’s sarcoma in organ transplant recipients. The Collaborative Transplantation Research Group of Ile de France. Eur J Med. 1993; 2:339–334.16. Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic venoocclusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003; 10:451–462.