Korean J Ophthalmol.
2013 Oct;27(5):345-350. 10.3341/kjo.2013.27.5.345.
Cataracts among Adults Aged 30 to 49 Years: A 10-Year Study from 1995 to 2004 in Korea
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Seoul, Korea. eyedoc@catholic.ac.kr
- KMID: 1707288
- DOI: http://doi.org/10.3341/kjo.2013.27.5.345
Abstract
- PURPOSE
To investigate the long-term characteristics of cataracts among adults aged 30 to 49 years in Korean over a span of 10 years.
METHODS
Subjects between the ages of 30 to 49 years who underwent cataract surgery at St. Mary's Hospital from 1995 to 2004 (n = 976) were included. Patients with a history of ocular trauma, uveitis, other ocular or systemic diseases, and congenital cataracts were excluded. Additional information including type of lens opacity, urban/rural region, and pre- and postoperative visual acuities were analyzed. Lens opacity grading was conducted using Lens Opacity Classification System III. The Cochran-Armitage proportion trend test was used to analyze vision changes with the passage of time.
RESULTS
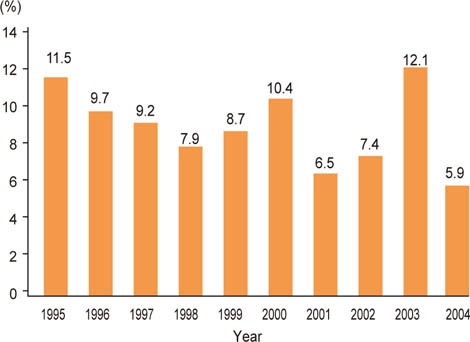
Among the patients who had undergone cataract surgeries, 8.8% (976 / 11,111) met the inclusion criteria. The mean age was 41.7 +/- 5.45 years. Gender breakdown of the patient population included 79.0% male and 21.0% female. In terms of home environment, 60.9% were from an urban region and 39.1% from a rural region. Opacity type included anterior polar (AP), posterior subcapsular (PSC), AP and PSC, cortical, and nuclear in 35.7%, 35.1%, 7.0%, 6.0%, and 5.4% of patients, respectively. At a 2-month postoperative follow-up appointment, 92.7% of patients showed a best-corrected visual acuity of more than 20 / 40.
CONCLUSIONS
Predominance of AP and PSC opacities as well as male patients was observed in this study population.
MeSH Terms
Figure
Reference
-
1. Hu TS, Zhen Q, Sperduto RD, et al. Age-related cataract in the Tibet Eye Study. Arch Ophthalmol. 1989; 107:666–669.2. Seah SK, Wong TY, Foster PJ, et al. Prevalence of lens opacity in Chinese residents of Singapore: the tanjong pagar survey. Ophthalmology. 2002; 109:2058–2064.3. West SK, Munoz B, Schein OD, et al. Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) project. Am J Epidemiol. 1998; 148:1033–1039.4. Leske MC, Connell AM, Wu SY, et al. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol. 1997; 115:105–111.5. Sperduto RD, Hiller R. The prevalence of nuclear, cortical, and posterior subcapsular lens opacities in a general population sample. Ophthalmology. 1984; 91:815–818.6. Adamsons I, Munoz B, Enger C, Taylor HR. Prevalence of lens opacities in surgical and general populations. Arch Ophthalmol. 1991; 109:993–997.7. Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population. The Beaver Dam Eye Study. Ophthalmology. 1992; 99:546–552.8. Mitchell P, Cumming RG, Attebo K, Panchapakesan J. Prevalence of cataract in Australia: the Blue Mountains eye study. Ophthalmology. 1997; 104:581–588.9. Xu L, Cui T, Zhang S, et al. Prevalence and risk factors of lens opacities in urban and rural Chinese in Beijing. Ophthalmology. 2006; 113:747–755.10. Tsai SY, Hsu WM, Cheng CY, et al. Epidemiologic study of age-related cataracts among an elderly Chinese population in Shih-Pai, Taiwan. Ophthalmology. 2003; 110:1089–1095.11. Husain R, Tong L, Fong A, et al. Prevalence of cataract in rural Indonesia. Ophthalmology. 2005; 112:1255–1262.12. Song KJ, Han MY, Kim SY, et al. Prevalence estimation of cataract based on a screening test. J Korean Ophthalmol Soc. 2007; 48:768–773.13. Yoon KC, Mun GH, Kim SD, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–433.14. Zetterström C, Lundvall A, Kugelberg M. Cataracts in children. J Cataract Refract Surg. 2005; 31:824–840.15. Jain IS, Pillay P, Gangwar DN, et al. Congenital cataract: etiology and morphology. J Pediatr Ophthalmol Strabismus. 1983; 20:238–242.16. Bardelli AM, Lasorella G, Vanni M. Congenital and developmental cataracts and multimalformation syndromes. Ophthalmic Paediatr Genet. 1989; 10:293–298.17. Cassidy L, Taylor D. Congenital cataract and multisystem disorders. Eye (Lond). 1999; 13(Pt 3b):464–473.18. Sachdev N, Tiakumzuk S, Aulakh R, Brar GS. Anomalous bilateral lateral rectus muscles and anterior polar cataract with dysmorphic features. J AAPOS. 2009; 13:319–321.19. Melamed J, Eckert GU, Spadoni VS, et al. Ocular manifestations of congenital toxoplasmosis. Eye (Lond). 2010; 24:528–534.20. Sharan S, Sharma S, Billson FA. Congenital rubella cataract: a timely reminder in the new millennium? Clin Experiment Ophthalmol. 2006; 34:83–84.21. Chylack LT Jr, Wolfe JK, Singer DM, et al. The lens opacities classification system III: the Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111:831–836.22. Kim H, Joo CK. The prevalence and demographic characteristics of anterior polar cataract in a hospital-based study in Korea. Korean J Ophthalmol. 2008; 22:77–80.23. Kim HJ, Park JW, Joo CK. An epidemiological study of the risk factors associated with anterior polar cataract. J Korean Ophthalmol Soc. 2003; 44:606–614.24. Majima K, Majima Y. Histopathological and cell biological analyses of the formation mechanism of anterior polar cataract. Ophthalmologica. 1999; 213:34–39.25. Hess R, Woo G. Vision through cataracts. Invest Ophthalmol Vis Sci. 1978; 17:428–435.26. Cinotti AA. Evaluation of indications for cataract surgery. Ophthalmic Surg. 1979; 10:25–31.27. Jaffe NS. Glare and contrast: indications for cataract surgery. J Cataract Refract Surg. 1986; 12:372–375.28. Koch DD. Glare and contrast sensitivity testing in cataract patients. J Cataract Refract Surg. 1989; 15:158–164.29. Neumann AC, McCarty GR, Steedle TO, et al. The relationship between cataract type and glare disability as measured by the Miller-Nadler glare tester. J Cataract Refract Surg. 1988; 14:40–45.30. Stifter E, Sacu S, Benesch T, Weghaupt H. Impairment of visual acuity and reading performance and the relationship with cataract type and density. Invest Ophthalmol Vis Sci. 2005; 46:2071–2075.31. Stifter E, Sacu S, Weghaupt H. Functional vision with cataracts of different morphologies: comparative study. J Cataract Refract Surg. 2004; 30:1883–1891.32. McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999; 128:446–465.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk of Depression Associated with Common Chronic Eye Diseases: A Study Using the 8th Korea National Health and Nutrition Examination Survey (2019–2021)
- Association between Vitamin D and Allergic Disease and Cataract in Korean Adults
- Prevalence and Clinical Characteristics of Dyslipidemia in Koreans
- Age classification for tooth loss management in Korean adults
- Factors Associated with Awareness, Treatment, and Control Rate of Hypertension among Korean Young Adults Aged 30–49 Years