J Korean Soc Radiol.
2011 Aug;65(2):119-125. 10.3348/jksr.2011.65.2.119.
Feasibility of Endovascular Radiation Therapy Using Holmium-166 Filled Balloon Catheter in a Swine Hemodialysis Fistula Model: Preliminary Results
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea. jywon@yuhs.ac
- 2Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Radiology, School of Medicine, Ewha Woman's University, Seoul, Korea.
- 4Department of Internal Medicine, School of Medicine, Ewha Woman's University, Seoul, Korea.
- KMID: 1443473
- DOI: http://doi.org/10.3348/jksr.2011.65.2.119
Abstract
- PURPOSE
To describe how to make a swine hemodialysis fistula model and report our initial experience to test the feasibility of endovascular radiation therapy with Holmium-166 filled balloon catheters.
MATERIALS AND METHODS
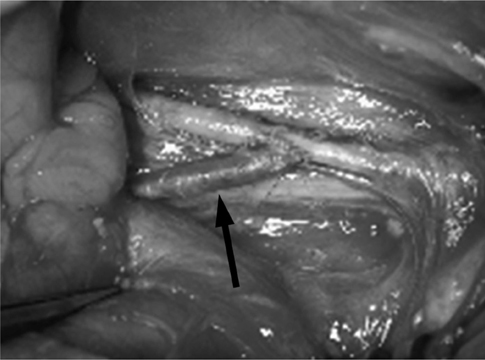
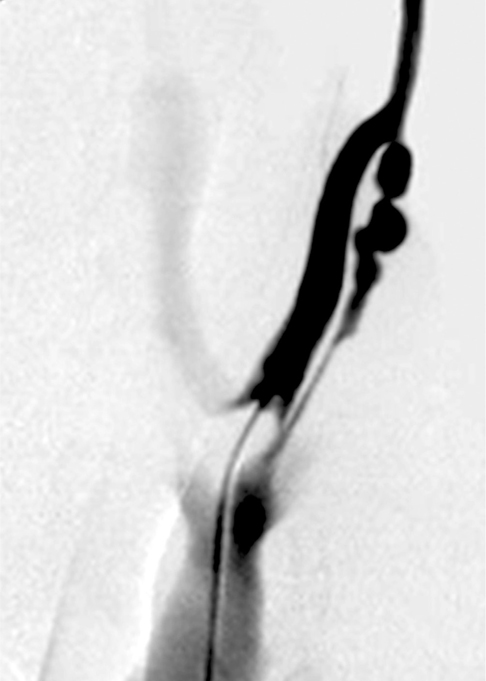
The surgical formation of arterio-venous fistula (AVF) was performed by end-to-side anastomosis of the bilateral jugular vein and carotid artery of 6 pigs. After 4 weeks, angiograms were taken and endovascular radiation was delivered to the venous side of AVF with Holmium-166 filled balloon catheters. Pigs were sacrificed 4 weeks after the radiation and AVFs were harvested for histological examination.
RESULTS
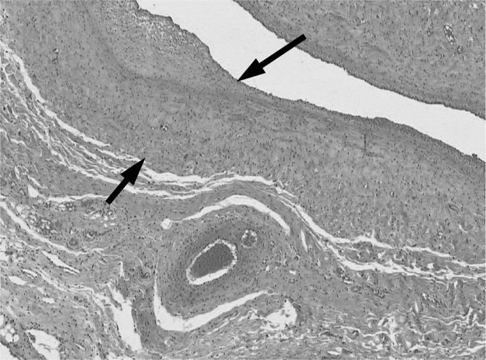
All animals survived without any morbidity during the experimental periods. The formation of fistula on the sides of necks was successful in 11 of the 12 pigs (92%). One AVF failed from the small jugular vein. On angiograms, 4 of the 11 AVFs showed total occlusion or significant stenosis and therefore, endovascular radiation could not be performed. Of 7 eligible AVFs, five underwent successful endovascular radiation and two AVFs did not undergo radiation for the control. Upon histologic analysis, one non-radiated AVF showed total occlusion and others showed intimal thickening from the neointimal hyperplasia.
CONCLUSION
Formation of the swine carotid artery-jugular vein hemodialysis fistula model was successful. Endovascular radiation using a Holmium-166 filled balloon catheter was safe and feasible.
MeSH Terms
Figure
Reference
-
1. Diskin CJ. Pharmacologic intervention to prevent hemodialysis vascular access thrombosis: the next generation of treatment? Kidney Int. 2005; 67:2505.2. Windus DW. Permanent vascular access: a nephrologist's view. Am J Kidney Dis. 1993; 21:457–471.3. Masaki T, Rathi R, Zentner G, Leypoldt JK, Mohammad SF, Burns GL, et al. Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney Int. 2004; 66:2061–2069.4. Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol. 1995; 6:851–855.5. Sarac TP, Riggs PN, Williams JP, Feins RH, Baggs R, Rubin P, et al. The effects of low-dose radiation on neointimal hyperplasia. J Vasc Surg. 1995; 22:17–24.6. Teirstein PS, Massullo V, Jani S, Russo RJ, Cloutier DA, Schatz RA, et al. Two-year follow-up after catheter-based radiotherapy to inhibit coronary restenosis. Circulation. 1999; 99:243–247.7. Liermann DD, Bauernsachs R, Schopohl B, Böttcher HD. Five year follow-up after brachytherapy for restenosis in peripheral arteries. Semin Interv Cardiol. 1997; 2:133–137.8. Johnson MS, McLennan G, Lalka SG, Whitfield RM, Dreesen RG. The porcine hemodialysis access model. J Vasc Interv Radiol. 2001; 12:969–977.9. Trerotola SO, Carmody TJ, Timmerman RD, Bergan KA, Dreesen RG, Frost SV, et al. Brachytherapy for the prevention of stenosis in a canine hemodialysis graft model: preliminary observations. Radiology. 1999; 212:748–754.10. Popma JJ, Suntharalingam M, Lansky AJ, Heuser RR, Speiser B, Teirstein PS, et al. Randomized trial of 90Sr/90Y beta-radiation versus placebo control for treatment of in-stent restenosis. Circulation. 2002; 106:1090–1096.11. Grise MA, Massullo V, Jani S, Popma JJ, Russo RJ, Schatz RA, et al. Five-year clinical follow-up after intracoronary radiation: results of a randomized clinical trial. Circulation. 2002; 105:2737–2740.12. Joh CW, Park CH, Kang HJ, Oh YT, Chun , Kim HS, et al. Measurement of radiation absorbed dose in endovascular Ho-166 brachytherapy using a balloon angio-catheter. Nucl Med Commun. 2000; 21:959–964.13. Kanterman RY, Vesely TM, Pilgram TK, Guy BW, Windus DW, Picus D. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology. 1995; 195:135–139.14. Roy-Chaudhury P, Kelly BS, Narayana A, Desai P, Melhem M, Munda R, et al. Hemodialysis vascular access dysfunction from basic biology to clinical intervention. Adv Ren Replace Ther. 2002; 9:74–84.15. Kim SJ, Masaki T, Leypoldt JK, Kamerath CD, Mohammad SF, Cheung AK. Arterial and venous smooth-muscle cells differ in their responses to antiproliferative drugs. J Lab Clin Med. 2004; 144:156–162.16. Rodriguez VM, Grove J, Yelich S, Pearson D, Stein M, Pevec WC. Effects of brachytherapy on intimal hyperplasia in arteriovenous fistulas in a porcine model. J Vasc Interv Radiol. 2002; 13:1239–1246.17. Malhotra S, Teirstein PS. The SCRIPPS trial--catheter-based radiotherapy to inhibit coronary restenosis. J Invasive Cardiol. 2000; 12:330–332.18. King SB 3rd, Williams DO, Chougule P, Klein JL, Waksman R, Hilstead R, et al. Endovascular beta-radiation to reduce restenosis after coronary balloon angioplasty: results of the beta energy restenosis trial (BERT). Circulation. 1998; 97:2025–2030.19. Waksman R, Robinson KA, Crocker IR, Gravanis MB, Cipolla GD, King SB 3rd. Endovascular low-dose irradiation inhibits neointima formation after coronary artery balloon injury in swine. A possible role for radiation therapy in restenosis prevention. Circulation. 1995; 91:1533–1539.20. Kim HS, Cho YH, Kim JS, Oh YT, Kang HJ, Chun MS, et al. Effect of transcatheter endovascular radiation with holmium-166 on neointimal formation after balloon injury in porcine coronary artery. J Nucl Cardiol. 2000; 7:478–483.21. Badal A, Kyprianou I, Badano A, Sempau J. Monte Carlo simulation of a realistic anatomical phantom described by triangle meshes: application to prostate brachytherapy imaging. Radiother Oncol. 2008; 86:99–103.22. Kwok CS, Prestwich WV, Wilson BC. Calculation of radiation doses for nonuniformity distributed beta and gamma radionuclides in soft tissue. Med Phys. 1985; 12:405–412.23. Kim JH, Lee JT, Kim EK, Won JY, Kim MJ, Lee JD, et al. Percutaneous sclerotherapy of renal cysts with a beta-emitting radionuclide, holmium-166-chitosan complex. Korean J Radiol. 2004; 5:128–133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bakri and Coda Balloon Placement between the Liver and Bowel in a Swine Model: A Feasibility Study
- Effects of Intraluminal Irradiation with Holmium-166 for TIPS Stenosis: Experimental Study in a Swine Model
- Rupture, Breakdown, and Pulmonary Artery Embolism of a Balloon Catheter Tip during Percutaneous Transluminal Angioplasty of Arteriovenous Fistula
- Undeflatable balloon guide catheter (BGC) during endovascular procedure: Rescue strategy
- Cervical spinal extradural arteriovenous fistula successfully treated using transarterial balloon-assisted coil embolization