J Korean Soc Radiol.
2012 Feb;66(2):139-147. 10.3348/jksr.2012.66.2.139.
Vascular Biocompatibility of a Triple Layered Self Expanding Stent-Graft in a Dog Model
- Affiliations
-
- 1Department of Radiology, Ajou University School of Medicine, Suwon, Korea. wonkwak@ajou.ac.kr
- 2Medical Science Research Center, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Radiology, Pusan National University Medical College, Busan, Korea.
- KMID: 1439405
- DOI: http://doi.org/10.3348/jksr.2012.66.2.139
Abstract
- PURPOSE
To evaluate performance and biocompatibility of a newly designed self-expanding stent graft, which consisted of two nitinol stents and an intervening expanded polytetrafluoroethylene membrane in a dog artery model.
MATERIALS AND METHODS
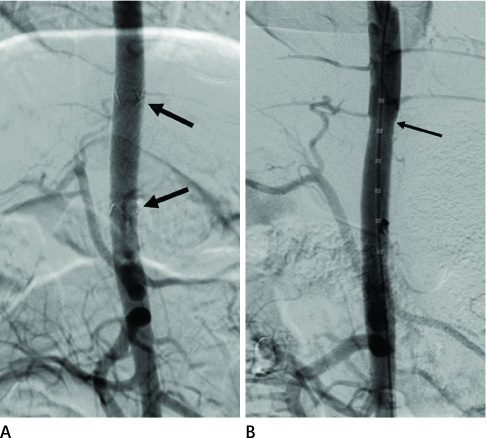
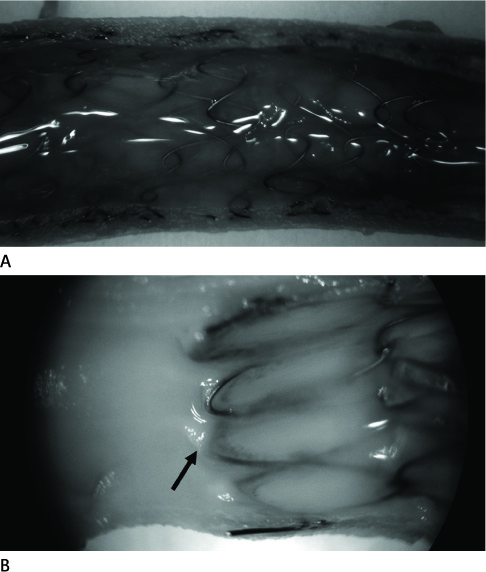
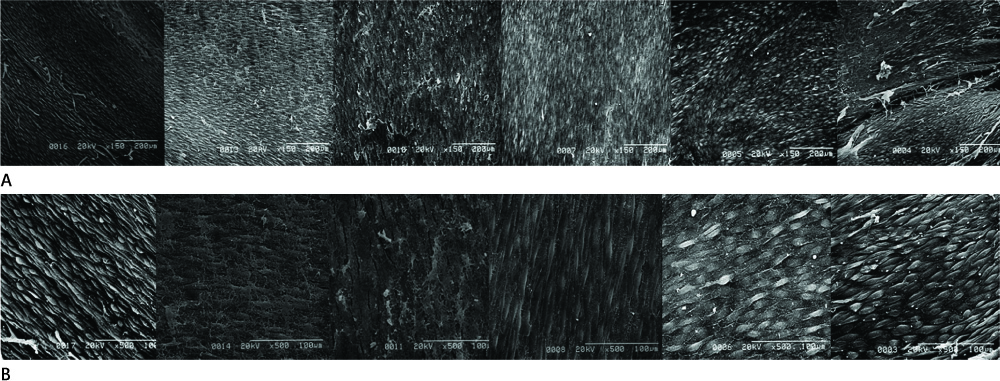
Twelve stent grafts were placed in the aorta of 6 dogs (beagle, mean body weight 11 kg) for 4 weeks (n = 4) and 12 weeks (n = 8). Luminal diameters were measured for each segment (the proximal bare, the middle graft, the distal bare) by angiographies after implantation and follow up periods. Percent luminal stenosis based on angiographies, histomorphometric, histologic, and scanning electron microscopic analyses of each segments were performed.
RESULTS
Blood flow through the stent grafts was good after implantation and during the follow up period, without thrombotic occlusion or stent graft migration. The mean percent luminal stenosis of the proximal bare, the middle grafted and the distal bare segments after 12 weeks were 13.5%, 3.9%, 9.6% retrospectively. The mean neointimal areas of the middle grafted segment were 4.39 mm2 (4 week) and 4.92 mm2 (12 week). Mature endothelialization was evident in over 70% of the area of the stented artery after 4 weeks and in over 90% after 12 weeks.
CONCLUSION
The stent graft was well placed in the attempted area without migration. During the 12-week-follow up period, it showed a good patency without thrombotic occlusion or significant in-stent luminal stenosis. Endothelialization was rapid and nearly complete. Neointima was thin and smooth on the middle graft segment and thicker and irregular on the bare segments.
MeSH Terms
Figure
Reference
-
1. Kaufman JA. Vascular interventions. In : Kaufman JA, Lee MJ, editors. Requisites vascular and interventional radiology. 1st ed. Philadelphia: Mosby;2004. p. 83–118.2. Curi MA, Geraghty PJ, Merino OA, Veeraswamy RK, Rubin BG, Sanchez LA, et al. Mid-term outcomes of endovascular popliteal artery aneurysm repair. J Vasc Surg. 2007; 45:505–510.3. Lee SM, Kang DH, Kim GH, Park WI, Kim HW, Park JH. Self-expanding metallic stents for gastric outlet obstruction resulting from stomach cancer: a preliminary study with a newly designed double-layered pyloric stent. Gastrointest Endosc. 2007; 66:1206–1210.4. Isayama H, Kawabe T, Nakai Y, Ito Y, Togawa O, Kogure H, et al. Management of distal malignant biliary obstruction with the ComVi stent, a new covered metallic stent. Surg Endosc. 2010; 24:131–137.5. Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc. 2007; 66:355–360.6. Cejna M, Virmani R, Jones R, Bergmeister H, Losert U, Xu Z, et al. Biocompatibility and performance of the Wallstent and several covered stents in a sheep iliac artery model. J Vasc Interv Radiol. 2001; 12:351–358.7. Cejna M, Virmani R, Jones R, Bergmeister H, Loewe C, Schoder M, et al. Biocompatibility and performance of the Wallstent and the Wallgraft, Jostent, and Hemobahn stent-grafts in a sheep model. J Vasc Interv Radiol. 2002; 13:823–830.8. Dolmatch BL, Dong YH, Trerotola SO, Hunter DW, Brennecke LH, LaBounty R. Tissue response to covered Wallstents. J Vasc Interv Radiol. 1998; 9:471–478.9. Virmani R, Kolodgie FD, Dake MD, Silver JH, Jones RM, Jenkins M, et al. Histopathologic evaluation of an expanded polytetrafluoroethylene-nitinol stent endoprosthesis in canine iliofemoral arteries. J Vasc Interv Radiol. 1999; 10:445–456.10. Jeremy JY, Thomas AC. Animal models for studying neointima formation. Curr Vasc Pharmacol. 2010; 8:198–219.11. Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998; 31:224–230.12. Yee DC, Williams SK, Salzmann DL, Pond GD, Patula V, Berman SS, et al. Stent versus endovascular graft healing characteristics in the porcine iliac artery. J Vasc Interv Radiol. 1998; 9:609–617.13. Ohki T, Marin ML, Veith FJ, Yuan JG, Ohki M, Soundararajan K, et al. Anastomotic intimal hyperplasia: a comparison between conventional and endovascular stent graft techniques. J Surg Res. 1997; 69:255–267.14. Tepe G, Duda SH, Hanke H, Schulze S, Hagmeier S, Bruck B, et al. Covered stents for prevention of restenosis. Experimental and clinical results with different stent designs. Invest Radiol. 1996; 31:223–229.15. Ombrellaro MP, Stevens SL, Kerstetter K, Freeman MB, Goldman MH. Healing characteristics of intraarterial stented grafts: effect of intraluminal position on prosthetic graft healing. Surgery. 1996; 120:60–70.16. Graham LM, Harrell KA, Sell RL, Crudup JW, Burkel WE, Stanley JC. Enhanced endothelialization of Dacron grafts by external vein wrapping. J Surg Res. 1985; 38:537–545.17. Cikirikcioglu M, Sedelnikov N, Osorio-Da Cruz S, Khabiri E, Donmez Antal A, Tatar T, et al. Improved neo-endothelialization of small diameter ePTFE grafts with titanium coating. Int J Artif Organs. 2006; 29:990–999.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanical Recanalization of Cerebral Artery Embolic Occlusion Using a Self-Expanding Stent: Experimental Analysis in Canine Model
- Self-Expandable Stenting over a Stent Graft for the Exclusion of a Carotid Stump: Troubleshooting for Device Incompatibility
- Development of New Coronary Stent-Grafts using Surface-Modified Polymers
- Axillary Artery Rupture after Shoulder Dislocation That Was Treated with a Self-Expanding Stent - A Case Report -
- Femoropopliteal Artery Stent Fracture with Recurrent In-Stent Reocclusion and Aneurysm Formation: Successful Treatment with Self-Expandable Viabahn Endoprosthesis